NIH will reinstate 900 grants in response to court order
On Jun. 25, 2025, the National Institutes of Health (NIH) moved to reinstate about 900 grants that a…

On Jun. 25, 2025, the National Institutes of Health (NIH) moved to reinstate about 900 grants that a…

On Jun. 11, 2025, a study led by by University of Barcelona experts Carles J. Ciudad and Verònica…
On Jul. 30, 2024, a head-to-head comparison of six commercially available blood tests for Alzheimer’s disease led by…

On Jun. 27, 2024, C2N Diagnostics (C2N), has entered into a non-exclusive agreement with Mayo Clinic Laboratories, for…

On Feb. 24, 2024, C2N Diagnostics (C2N) announced the medical device registration from the United Kingdom’s Medicines and…

On Nov. 22, 2023, the U.S. Food and Drug Administration (FDA) issued the final guidance COVID-19: Developing Drugs…

On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…
On Dec. 5, 2022, the Fred Hutchinson Cancer Center announced that according to a new meta-analysis women were…

On Nov. 15, 2022, Moderna reported findings from a Phase II/III clinical trial where bivalent Omicron-targeting booster candidates,…

On Nov. 12, 2021, Innovation Pharma announced the Company was conducting full data analysis of the Phase 2…

On Jun. 8, 2021, Mayo Clinic researchers and colleagues at the University of Minnesota showed that COVID-19 exacerbates…

On Nov. 30, 2020, Moderna announced that the primary efficacy analysis of the Phase 3 study of mRNA-1273…

On Nov. 18, 2020, Gilead Sciences announced topline results from the Phase 2/3 CAPELLA trial evaluating the company’s…

On Sept. 30, 2020, the National Institutes of Health (NIH) announced funding of a nationwide study to discover…

On Aug. 3, 2020, Atossa Therapeutics announced that it had received approval from the ethics committee to open…

On Apr. 6, 2020, the National Cancer Institute awarded of $14.54 million to Roswell Park Comprehensive Cancer Center…
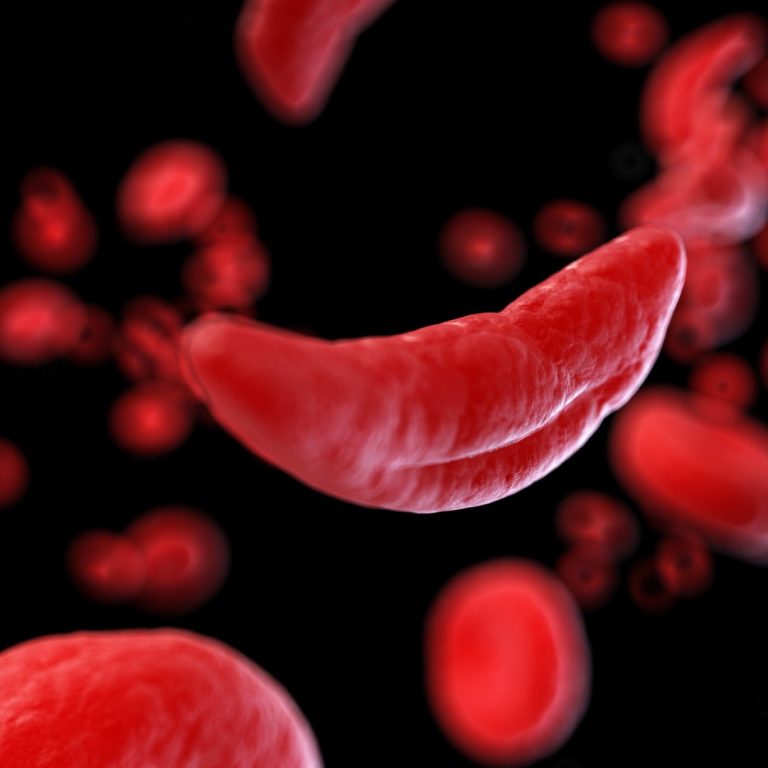
On Dec. 2, 2019, the U.S. Food and Drug Administration (FDA) announced it had approved the drug crizanlizumab…

On Nov. 27, 2018, the Canadian Cancer Trials Group, a new clinical trial, opened across Canada. This was…

On Aug. 29, 2016, Scripps Health and MD Anderson Cancer Center announced a partnership agreement to create Scripps…
On Sept. 24, 2009, the second Phase III HIV-1 vaccine trial, also known as RV144, that began in…

On Jul. 22, 1993, revising a policy from 1977 that excluded women of childbearing potential from early drug…

In 1993, the Prostate, Lung, Colorectal, and Ovarian trial was launched. The trial was designed to determine whether…

In 1992, the National Cancer Institute (NCI) awarded the Pacific Health Research Institute its largest and most enduring…

In 1991, the American Association of Clinical Endocrinologists (AACE) was founded by a visionary group of leaders to…

On Jun. 25, 1988, the U.S. Postal Service (USPS) proposed to ban mailings of microbe samples capable of…

In 1988, the U.S. Food and Drug Administration (FDA) published a final rule, Annual Summary Reporting Requirements Under the…
In 1974, ground was broken on The Scripps Clinic and Research Foundation’s new site on the Torrey Pines…

In 1974, Albert Einstein Cancer Center’s Liver Research Center — now the interdisciplinary Marion Bessin Liver Research Center…

In 1973, the Ohio State University Cancer Center (OSUCCC) was established in Columbus. The patient care arm of…
In 1972, the Red Cross called for a national blood policy to support standardized practices and end paid…