Quest Diagnostics launched Monkeypox virus testing
On Jul. 13, 2022, Quest Diagnostics announced that it had announced the nationwide availability* of the company’s lab-developed…
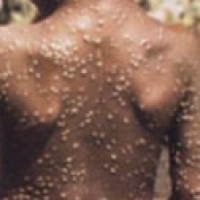
On Jul. 13, 2022, Quest Diagnostics announced that it had announced the nationwide availability* of the company’s lab-developed…

On Jul 13. 2022, Quest Diagnostics announced the nationwide availability of the company’s lab-developed molecular diagnostic test to…

On Jul. 6, 2022, LabCorp announced that it had begun testing for monkeypox using the U.S. Centers for…

On Jun. 10, 2022, CDC and public health officials in several states announced they were investigating multistate outbreaks…
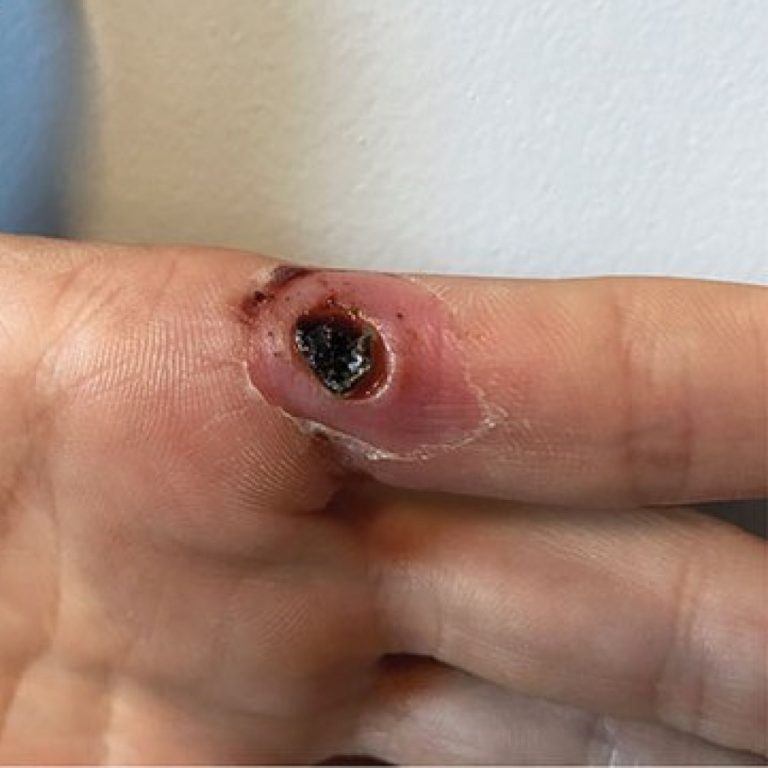
On May 18, 2022, scientists at the Centers for Disease Control and Prevention (CDC) announced that they were…

On May 6, 2022, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) confirmed the…

On April 21, 2022, researchers at the Centers for Disease Control and Prevention’s (CDC) National Institute for Occupational…

On Mar. 23, 2022, Quest Diagnostics announced that it had been granted a contract by the Centers for…
On Mar. 11, 2022, the BNT162b2 (Pfizer-BioNTech) mRNA COVID-19 vaccine was recommended by CDCメs Advisory Committee on Immunization…

On Mar. 4, 2022, the CDC reported that COVID-19 vaccination coverage with the first dose of the primary…
On Mar. 1, 2022, the CDC reported that Two doses of Pfizer-BioNTech vaccine protect against COVID-19ヨassociated emergency department…

On Feb. 4, 2022, the Advisory Committee on Immunization Practices (ACIP) issued a standard recommendation for use of…

On Dec. 7, 2021, the Centers for Disease Control and Prevention announced that it had awarded $22 million…

On Dec. 1, 2021, the California and San Francisco Departments of Public Health confirmed that a recent case…

On Nov. 17, 2021, scientists at the Centers for Disease Control and Prevention (CDC) announced they were collaborating…
On Oct. 29, 2021, the Centers for Disease Control and Prevention (CDC) published “new science” reinforcing that vaccination…

On Oct. 22, 2021, the U.S. Centers for Disease Control and Prevention (CDC) reported two new U.S. human…

On Oct. 21, 2021, Johnson & Johnson announced that the U.S. Centers for Disease Control and Prevention (CDC)…

On Oct. 7, 2021, the Centers for Disease Control and Prevention (CDC) reported One U.S. child loses a…

On Sept. 17, 2021, the Centers for Disease Control and Prevention (CDC) announced a $2.1 billion investment to…

On Sept. 3, 2021, the Centers for Disease Control and Prevention (CDC) awarded more than $116 million in…
On Aug. 6, 2021, the Centers for Disease Control and Prevention (CDC) annouced in MMWR that a study…

On Aug. 4, 2021, Bio-Techne announced that scientists at the U.S. Centers for Disease Control and Prevention (CDC)…

On Jul. 16, 2021, the U.S. Centers for Disease Control and Prevention (CDC) announced and the Texas Department…

On Jun. 8, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved PREVNAR 20ル…

On Apr. 28, 2021, the Centers for Disease Control and Prevention (CDC) announced that both mRNA COVID-19 vaccines…

COVID-19 and it’s naysayers are attacking Science and Reason. The defenders must suffer the slings and arrows from…

On Apr. 23, 2021, following a thorough safety review, including two meetings of the CDCメs Advisory Committee on…

As of Apr. 12, 2021, more than 6.8 million doses of the Johnson & Johnson (Janssen) vaccine were…

On Apr. 13, 2021, the FDA and CDC announced that they were are reviewing data involving six reported…