Long-acting injectable drug prevented HIV in cisgender women
On Nov. 9, 2020, the NIH reported that a pre-exposure prophylaxis (PrEP) regimen containing an investigational long-acting form…
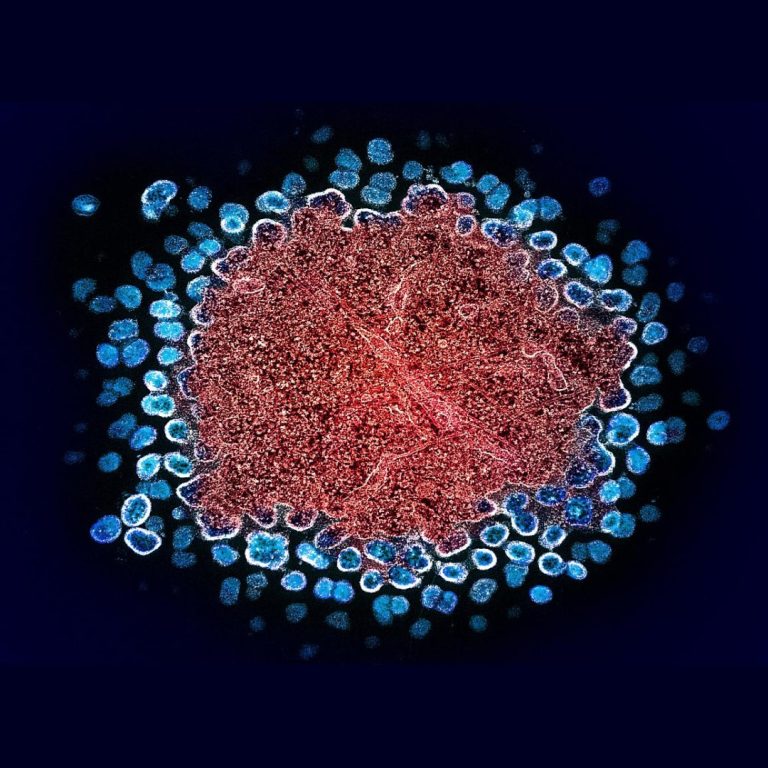
On Nov. 9, 2020, the NIH reported that a pre-exposure prophylaxis (PrEP) regimen containing an investigational long-acting form…
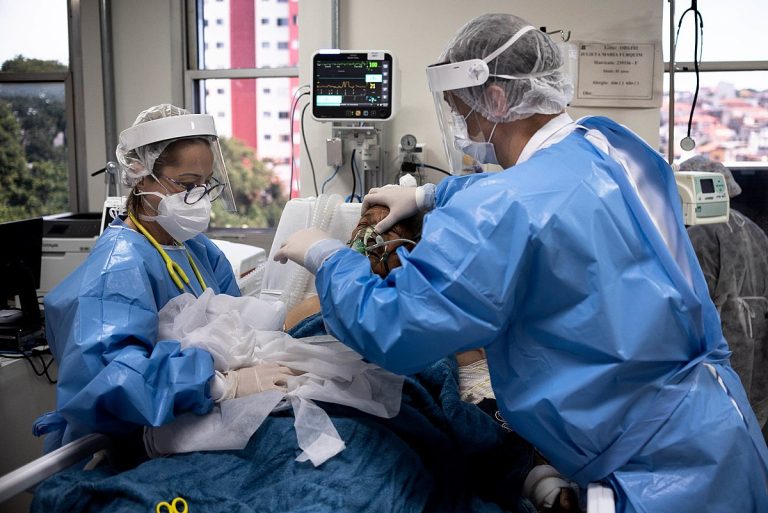
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…

On Nov. 9, 2020, a study published in JAMA reported the effect of hydroxychloroquine on clinical status at…

On Nov. 8, 2020, research from the U.S. had shown that white-tailed deer were being infected with SARS-CoV-2,…

On Nov. 6, 2020, Mesa Biotech announced it had been awarded a contract up to $13 million from…

On Nov. 6, 2020, Humanigen announced that it had entered into a Cooperative Research and Development Agreement (CRADA)…

On Nov. 6, 2020, Thermo Fisher Scientific and Innoforce announced a joint venture agreement to establish a new…
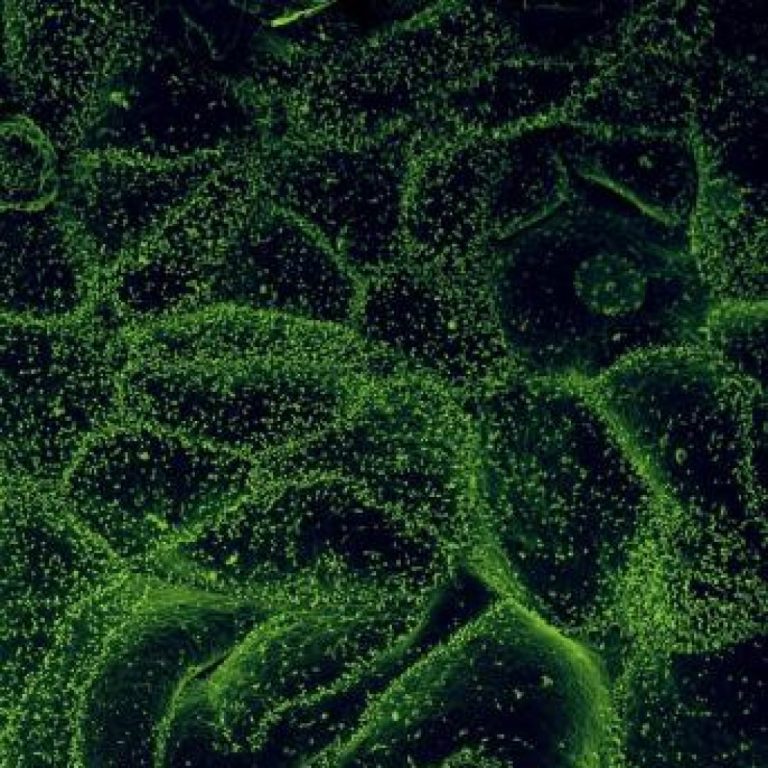
On Nov. 6, 2020, in a study published in Cell, the researchers identified antiviral defense genes that the…

On Nov. 5, 2020, Denmark announced strict lockdown rules in the north of the country after authorities discovered…

On Nov. 4, 2020, Mateon Therapeutics announced the receipt of approval from Instituto Nacional de Salud (INS), the…

On Nov. 4, 2020, Novavax announced the signing of a non-binding Heads of Terms document with the Australian…

On Nov. 4, 2020, McGill University Professor William Foulkes awarded 2020 Wilder-Penfield Prize for research in the genetics…
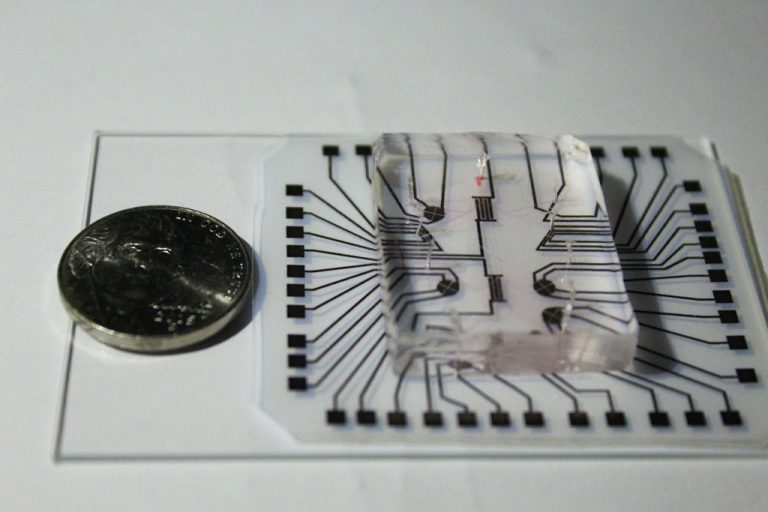
On Nov. 4, 2020, using the cutting-edge genetic editing technique known as CRISP ‘lab on a chip’ technology,…

On Nov. 3, 2020, a study published in JAMA Network Open showed that children from poorer neighborhoods performed…

On Nov. 3, 2020, Medigen Vaccine Biologics (MVC) and the National Institute of Hygiene and Epidemiology (NIHE), a…

On Nov. 3, 2020, OraSure Technologies announced its DNA Genotek subsidiary has received Emergency Use Authorization from the…

On Nov. 2, 2020, Novavax announced the expansion of its Maryland campus to accommodate the company’s rapid growth…

On Nov. 2, 2020, Pacific Northwest National Laboratory (PNNL) announced that in the Spring of 2020, as news…

On Nov. 1, 2020, the U.S. Department of Defense announced the start of rapid, on-site COVID-19 testing for…
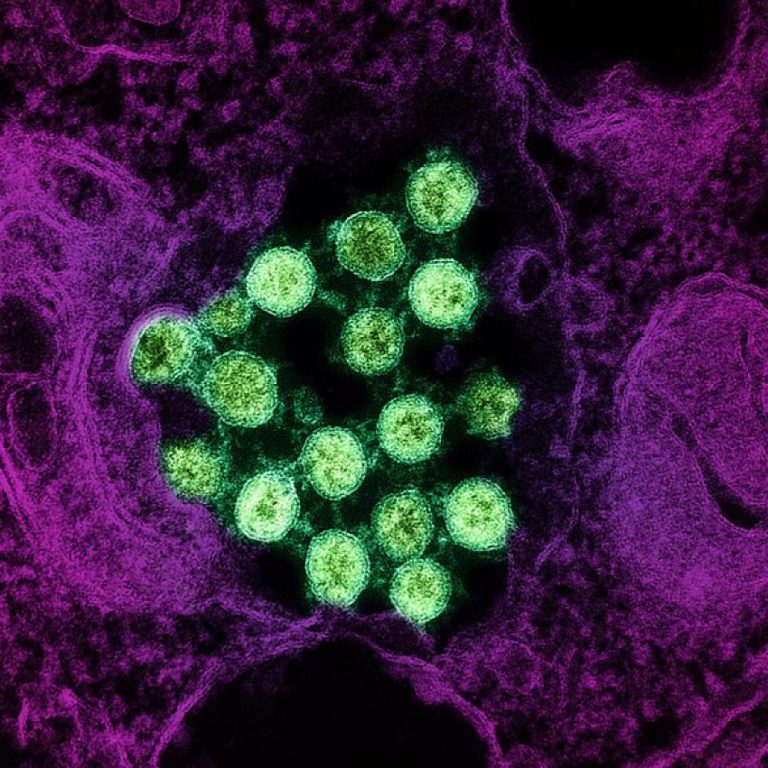
On Nov. 1, 2020, scientists announced that increasing evidence shows that both natural and synthetic antimicrobial peptides (AMPs),…

On Oct. 30, 2020, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced enrollment of volunteers in a phase…

On Oct. 30, 2020, the U.S. Department of Health and Human Services (HHS) and the U.S. Department of…
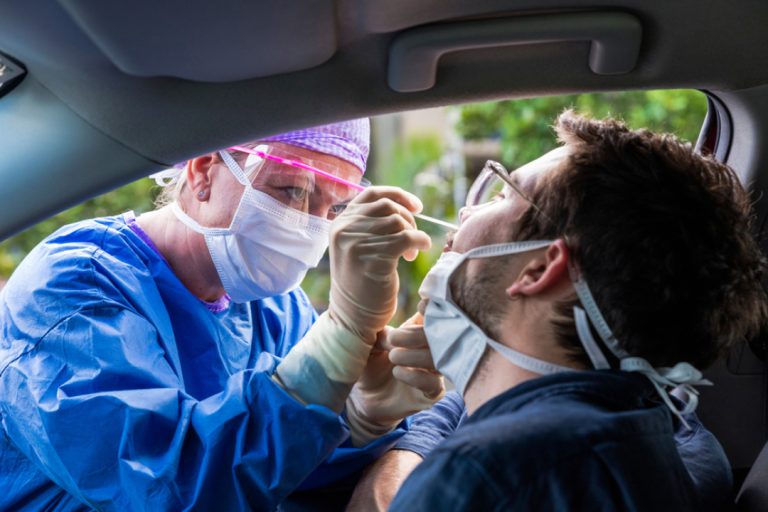
On Oct. 29, 2020, Mammoth Biosciences announced that it had signed agreements with MilliporeSigma and Hamilton Company targeting…

On Oct. 29, 2020, AquaBounty Technologies announced that it had identified Mayfield, Kentucky as the potential location for…

On Oct. 29, 2020, Takeda Pharmaceutical announced that it would import and distribute 50 million doses of Moderna’s…

On Oct. 29, 2020, the University of California, Berkeley (UC Berkeley) announced that it had launched a new…

On Oct. 28, 2020, Eli Lilly announced an initial agreement with the U.S. government to supply 300,000 vials…
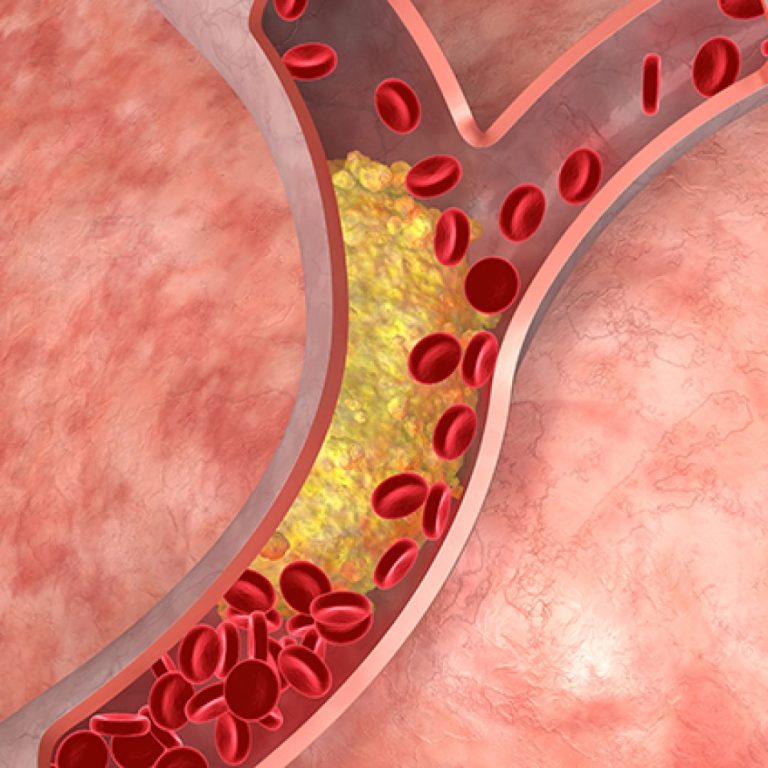
On Oct. 28, 2020, University of Texas (UT) Southwestern scientists reported that an X-ray test commonly used to…

On Oct. 28, 2020, Seagen announced the closing of a $1.0 billion equity investment by Merck in 5.0…

On Oct. 27, 2020, researchers from the National Institutes of Health (NIH) announced they had discovered a new…