Moderna announced FDA authorization of booster dose of COVID-19 vaccine in the U.S.
On Oct. 20, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency…

On Oct. 20, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency…

On Oct. 20, 2021, Quest Diagnostics announced it had formed an agreement with the Texas Department of State…
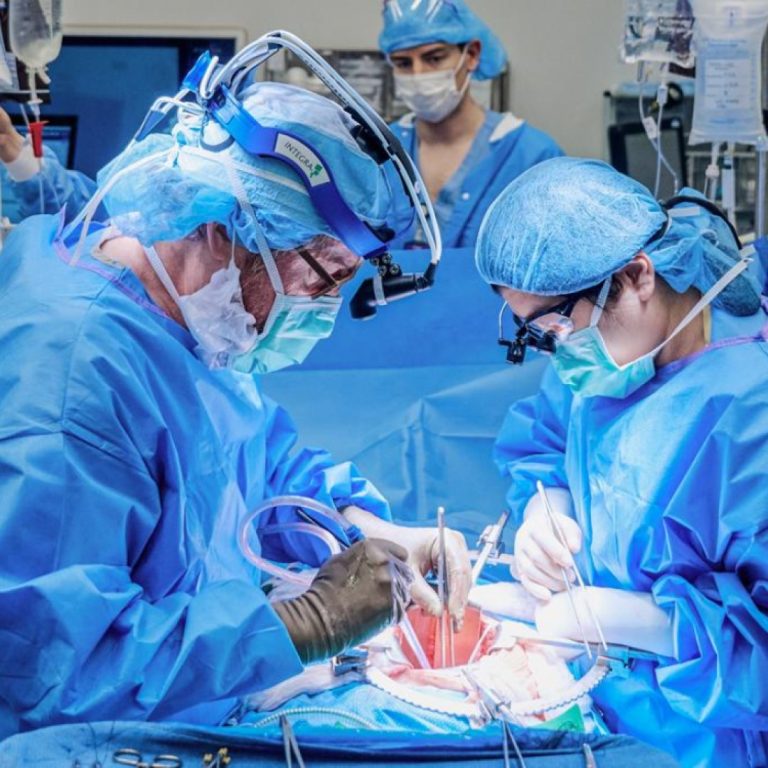
On Oct. 19, 2021, the first investigational transplant of a genetically engineered, nonhuman kidney to a human body…

On Oct. 18, 2021, Gilead Sciences announced that the Food and Drug Administration had approved a new low-dose…

On Oct. 18, 2021, Athenex and the Center for Cell and Gene Therapy at Baylor College of Medicine,…

On Oct. 18, 2021, Pfizer and BioNTech announced that the European Medicines Agency’s Committee for Human Medicinal Products…

On Oct. 15, 2021, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Tecentriq (atezolizumab)…

On Oct. 15, 2021, Pfizer and BioNTech announced they had submitted data supporting the vaccination of children 5…

On Oct. 14, 2021, Moderna confirmed that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological…

On Oct. 13, 2021, the World Health Organization (WHO) honoured the late Henrietta Lacks with a WHO Director-General’s…

On Oct. 12, 2021, Lucira Health announced that it had received PSAR approval for its Lucra CHECK IT…

On Oct. 12, 2021, Moderna announced that Gavi, the Vaccine Alliance had exercised its option to purchase an…

On Oct. 12, 2021, Inovio Pharmaceuticals announced online preprint publication in MedRxiv of Phase 1 clinical data on…

On Oct. 11, 2021, scientists at Washington University School of Medicine in St. Louis announced they had developed…

On Oct. 11, 2021, Inovio Pharmaceuticals announced that it had received authorization from Colombia’s INVIMA, to conduct the…
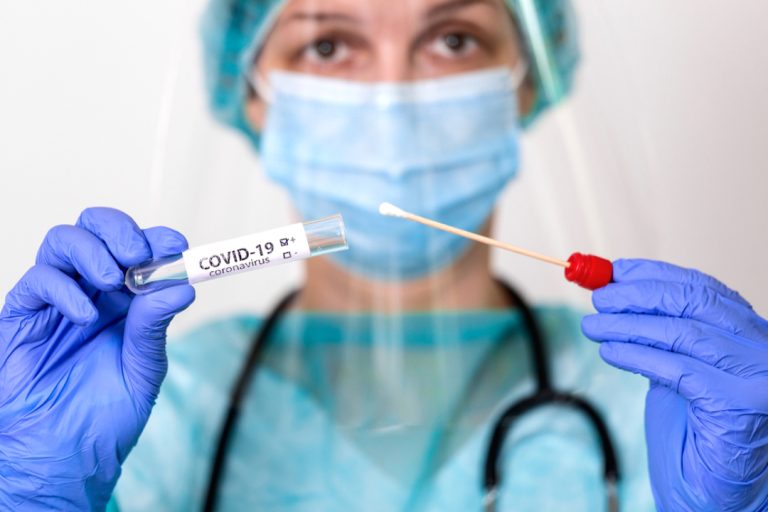
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Oct. 7, 2021, Vaxart announced that a Duke University-led study published in bioRxiv showed that Vaxart’s (investigational…

On Oct. 7, 2021, Moderna announced it was building a state-of-the-art mRNA facility in Africa with the goal…

On Oct. 6, 2021, Washington University School of Medicine announced the start of a pediatric COVID-19 vaccine clinical…

On Oct. 5, 2021, Moderna announced that the European Medicines Agency (EMA) had authorized a third dose of…

On Oct. 4, 2021, the Fred & Pamela Buffett Cancer Center announced it had successfully renewed its National…

The earliest physical trace of beer dates back to the late fourth millennium BC in present day Iran…

Around 600 BCE, Chinese healers created the first antibiotic – moldy soybean curds – to treat boils.

Around 500 BCE, Hippocrates, a pioneering physician in the history of Medicine, and considered as the principal author…

Around 100 BCE, Chinese growers used powdered chrysanthemum (pyrethrins) as the first insecticide.

On Oct. 4, 2021, Dynavax Technologies and the U.S. Department of Defense (DOD) announced Dynavax had executed an…

On Oct. 4, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

World’s oldest evidence dating to 23000 BCE for the earliest small-scale plant cultivation was discovered by Dani Nadel…

Around 8000 BCE, farmers in China, Egypt and Sumeria began using fermentation to produce cheese and wine. One…

Earliest known evidence of cheese making was found in Croatia dated to 7200 BCE. Residues from sherds of…