‘Ultrapotent’ antibodies neutralized SARS-CoV-2 variants in NIAID study
On Jan. 20, 2022, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) announced that they…

On Jan. 20, 2022, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) announced that they…

On Jan. 20, 2022, a clinical trial funded by the National Institutes of Health (NIH) found that giving…

On Jan. 19, 2022, Novavaxa announced that Australia’s Therapeutic Goods Administration had granted approval for provisional registration of…

On Jan. 18, 2022, Merck and Ridgeback Biotherapeutics announced the signing of a long-term supply agreement with the…

On Jan. 14, 2022, the U.S. Department of Agriculture’s (USDA) National Veterinary Services Laboratories confirmed a highly pathogenic…

On Jan. 14, 2022, the U.S. Department of Defense, in coordination with the U.S. Department of Health and…

On Jan. 12, 2022, Novavax and SK bioscience, a vaccine business subsidiary of Korea-based SK Group, announced that…

On Jan. 12, 2022, Pfizer announced positive top-line results from a Phase 3 study describing the safety and…

On Jan. 11, 2022, GlaxoSmithKline and Vir Biotechnology announced the US Government had purchased an additional 600,000 doses…
On Jan. 10, 2022, Novavax and Serum Institute of India announced a regulatory submission to the South African…

On Jan. 6, 2022, two new studies published in the journal Current Biology showed that environmental DNA (eDNA)…
On Jan. 5, 2022, Pfizer and BioNTech announced a new research, development and commercialization collaboration to develop a…

On Jan. 4, 2022, Pfizer announced that the U.S. government had committed to purchasing an additional 10 million…

On Jan. 4, 2022, Tonix Pharmaceuticals announced an exclusive option agreement and research collaboration with Kansas State University…

On Dec. 29, 2021, the Department of Defense (DoD), on behalf of and in coordination with the U.S….

On Dec. 28, 2021, Novavax and Serum Institute of India announced that the Drugs Controller General of India…
On Dec. 28, 2021, Pacific Biosciences announced that its new HiFiViral SARS-CoV-2 solution had successfully sequenced and captured…

On Dec. 27, 2021, Moderna announced a revised supply agreement with the government of South Korea for 20…
On Dec. 27, 2021, Sorrento Therapeutics announced that initial testing of COVISTIX on recombinant N proteins demonstrated its…

On Dec. 27, 2021, Moderna announced that the Swiss Federal Government had exercised its option to purchase an…

Our Heroes and Remembrance illustration has Maurice Ralph Hilleman and John Enders, pioneering developers of common vaccines, and…

On Dec. 24, 2021, Merck and Ridgeback Biotherapeutics announced that Japanメs Ministry of Health, Labor and Welfare had…

On Dec. 23, 2021, Novavax and SK bioscience announced the expansion of the companies’ collaboration and license agreements…

On Dec. 23, 2021, Merck and Ridgeback Biotherapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Dec. 23, 2021, Pfizer and BioNTech announced that they had submitted longer-term follow-up data from the companiesメ…

On Dec. 22, 2021, Gilead Sciences announced full results from a Phase 3 investigational study evaluating the efficacy…
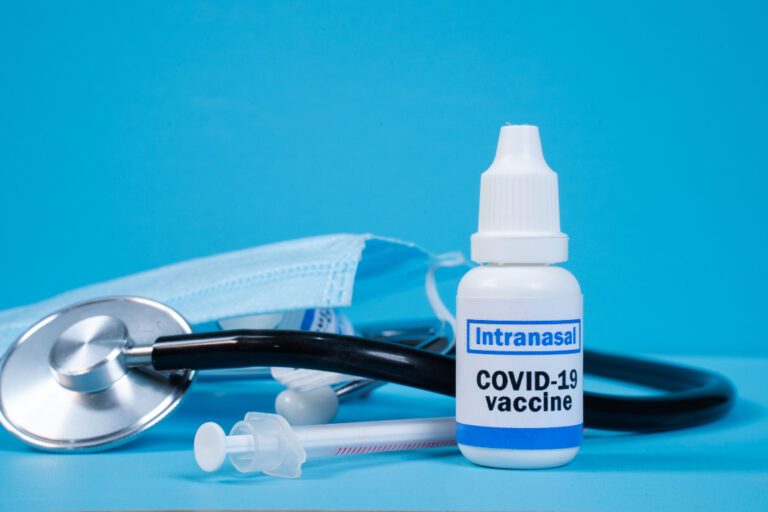
On Dec. 22, 2021, Cocrystal Pharma announced that in vitro studies demonstrate its oral and intranasal/pulmonary SARS-CoV-2 main…

On Dec. 22, 2021, Novavax announced initial data evaluating the immune response of its COVID-19 vaccine, NVX-CoV2373, against…

On Dec. 22, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…

On Dec. 21, 2021, Novavax announced that the first booster doses of NVX-CoV2373, the company’s recombinant nanoparticle protein-based…