Hundreds of new primate genomes offer window into human health and our past
On Jun. 1, 2023, two international teams announced they have sequenced the genomes of more than 200 nonhuman…

On Jun. 1, 2023, two international teams announced they have sequenced the genomes of more than 200 nonhuman…

Milestones in Parkinson’s illustrates advancements in the disease from James Parkinson’s essay on ‘shaking palsy’ to development of…

On May 31, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $10 million to five facilities as…

On May 30, 2023, the United Kingdom of Great Britain and Northern Ireland reported to the World Health…
On May 26, 2023, the University of Gothenburg announced that for the first time worldwide, in yet another…

On May 26, 2023, Novavax announced that Nuvaxovid (NVX-CoV2373) had been recommended for full Marketing Authorization (MA) for…

On May 25, 2023, the U.S. Food and Drug Administration (FDA) approved the oral antiviral Paxlovid (nirmatrelvir tablets…

On May 24, 2023, the U.S. FDA approved Braeburn pharmaceutical’s Brixadi (buprenorphine) extended-release injection for subcutaneous use (under…

On May 19, 2023, the U.S. Food and Drug Administration (FDA) cleared the Beta Bionics iLet ACE Pump…
On May 19, 2023, the U.S. Food and Drug Administration (FDA) approved Krystal Biotech’s Vyjuvek, a herpes-simplex virus…

On May 15, 2023, Brazil, the world’s top chicken exporter, for the first time confirmed Highly Pathogenic Avian…

On May 11, 2023, the U.S. Departments of Agriculture Animal (USDA) and Plant Health Inspection Service (APHIS) announced…

On May 11, 2023, the U.S. Food and Drug Administration (FDA) announced the supplemental approval of Otsuka Pharmaceutical…

On May 10, 2023, researchers released a new high-quality collection of reference human genome sequences that captures substantially…
On May 9, 2023, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
On May 8, 2023, scientists at the National Institutes of Health (NIH) announced that they had identified new…

On May 3, 2023, Washington State University (WSU) made history in the United States (US) by becoming the…
On May 3, 2023, the U.S. FDA approved GlaxoSmithKline’s Arexvy as the first respiratory syncytial virus (RSV) vaccine…
On May 3, 2023, National Institutes of Health (NIH) scientists announced that they had uncovered new details on…
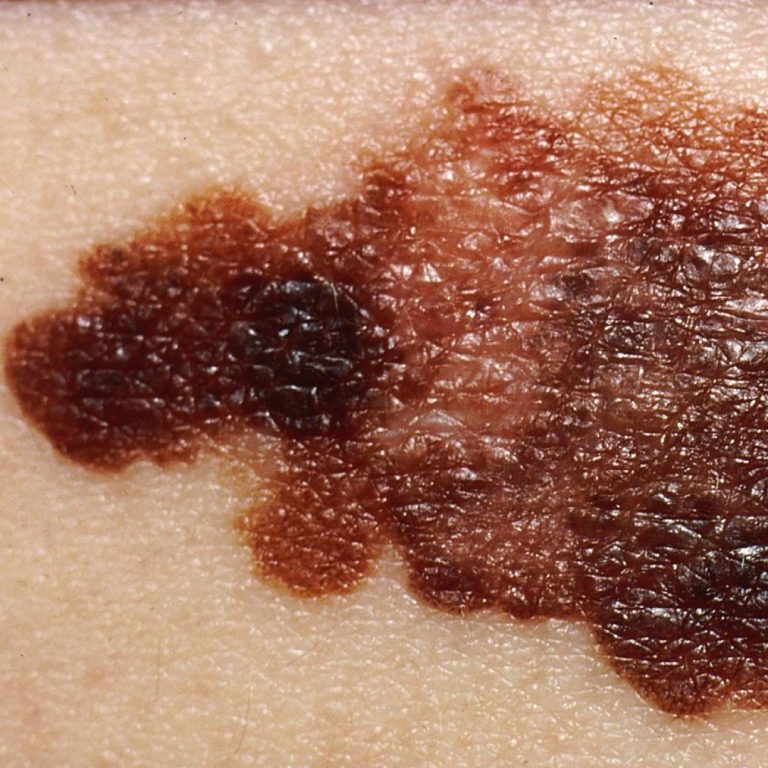
On May 1, 2023, an Oregon Health Sciences University (OHSU) dermatologist and a multi-disciplinary team confirmed that a…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…

On Apr. 27, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…

On Apr. 27, 2023, the Zoonomia Project team announced in in a special issue of Science, that had…

On Apr. 27, 2023, researchers at Cornell University announced they had used ancient DNA extraction and analysis to…
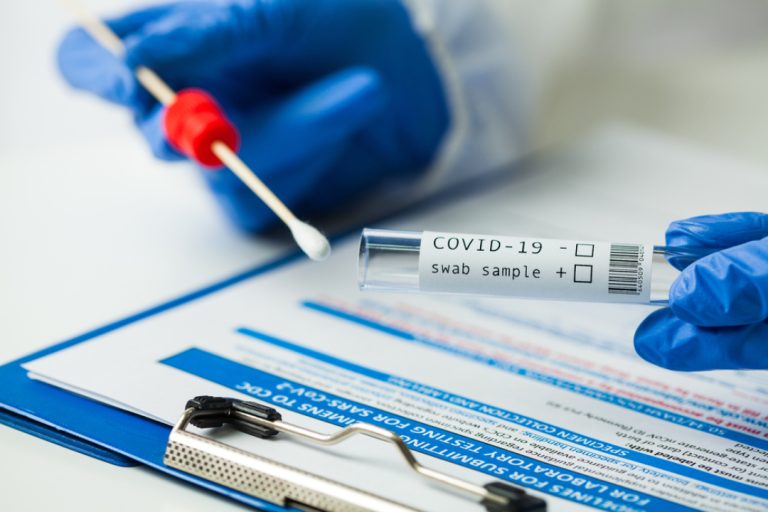
On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 20, 2023, Massachusetts Institute of Technology (MIT) researchers announced that have developed machine-learning algorithms that can…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Apr. 17, 2023, a new initiative led by Jennifer Doudna and Jill Banfield at the Innovative Genomics…

On Apr. 14, 2023, Revance Therapeutics announced that the U.S. Food and Drug Administration (FDA) had approved the…