Brain cells remain healthy after month on International Space Station, but matured faster than brain cells on Earth
On Dec. 16, 2024, Scripps Research scientists announced they had discovered how brain cells respond to microgravity. Scripps…

On Dec. 16, 2024, Scripps Research scientists announced they had discovered how brain cells respond to microgravity. Scripps…
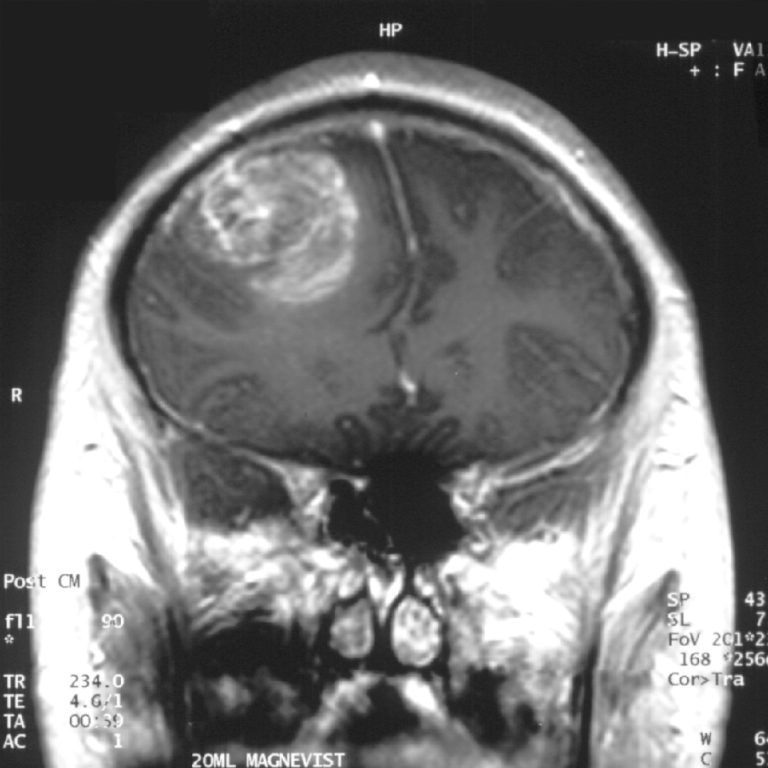
On Dec. 16, 2024, the Mayo Clinic announced the results of an innovative treatment approach that may offer…

On Dec. 16, 2024, JAMA Network Open published a study demonstrating respiratory syncytial virus (RSV) vaccine effectiveness in adults…

On Dec. 16, 2024, a study published in BMC Cardiovascular Disorders shows that pediatric and young adult COVID-19…

On Dec. 13, 2024, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced $165…

On Dec. 12, 2024, University of Pittsburgh researchers announced they had identified evidence of H5N1 adaptation in domestic…

On Dec. 12, 2024, the Oregon Department of Agriculture (ODA), with significant input from Oregon’s dairy industry and…

On Dec. 12, 2024, a study led by scientists from the Max Planck Institute for Evolutionary Anthropology in…

On Dec. 12, 2024, a study co-lead by researchers from the University of British Columbia, Vancouver General Hospital…

On Dec. 11, 2024, Massachusetts General Hospital (MGH), founding member of the Mass General Brigham healthcare system, announced…

On Dec. 11, 2024, University of Pennsylvania Engineers announced they had made a critical breakthrough that bridges a…

On Dec. 11, 2024 the Board of Directors of AquaBounty Technologies announced that the Company was winding down…
On Dec. 11, 2024, the World Health Organization (WHO) reported new data has revealed that an estimated 2.2…

On Dec. 10, 2024, researchers reported that DNA recovered from ancient remains is transforming our understanding of organisms…

On Dec. 10, 2024, the Pan American Health Organization (PAHO) reported on three transmissible diseases affecting the Region…

On Dec. 10, 2024, a research team led by Fangqiong Ling, an assistant professor at Washington University in…
On Dec. 9, 2024, neurologists at Fudan University in Shanghai, China have identified 13 proteins in the blood…

On Dec. 9, 2024, the Africa Centers for Disease Control (CDC) announced that it is working closely with…

On Dec. 6, 2024, the Arizona Department of Health Services reported the first human H5N1 cases in Arizona….

On Dec. 6, 2024, Shi Zhengli, the Chinese virologist at centre of COVID lab-leak theory, has presented evidence…

On Dec. 6, 2024, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) announced…

On Dec. 5, 2024, a study led by scientists at Scripps Research reveals that a single mutation in…
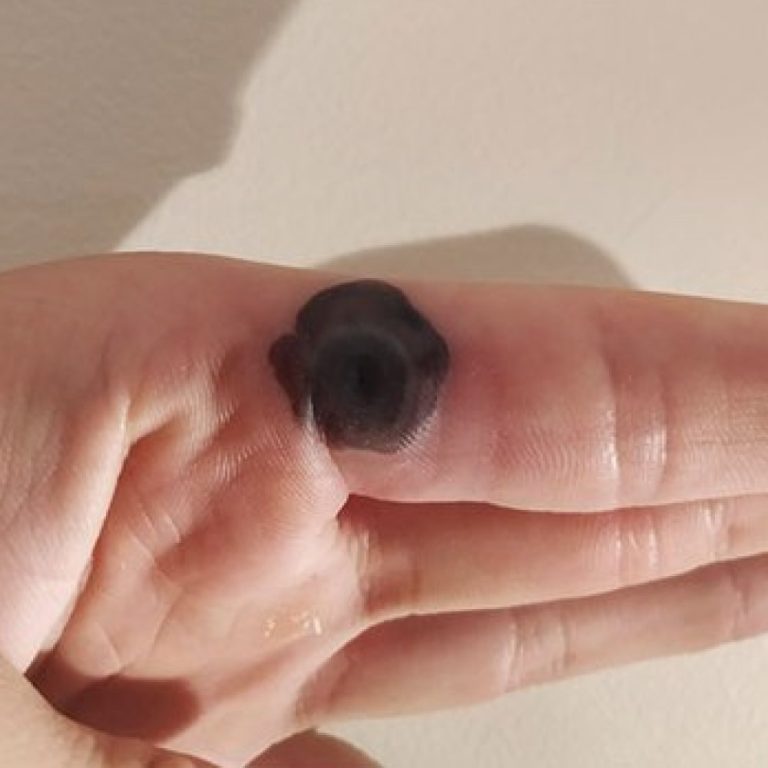
On Dec. 5, 2024, the World Health Organization (WHO) announced that as of December 1, 20 countries have…

On Dec. 5, 2024, the California Department of Public Health (CDPH) reported that following an investigation with the…

On Dec. 5, 2024, researchers Dr Sonia Shah and Dr Clara Jiang from the University of Queensland’s Institute…
On Dec. 5, 2024, the World Health Organization (WHO) has granted prequalification to the molecular diagnostic test for…

On Dec. 4, 2024 a study led by researchers from TGen and Arizona State University showed that pre-clinical models exposed…

On Dec. 4, 2024, The U.S. Food and Drug Administration (FDA) announced approved of AstraZeneca’s blockbuster drug Imfinzi…

On Dec. 4, 2024, artificial intelligence (AI) researchers, in an effort to automate scientific discovery announced they have…
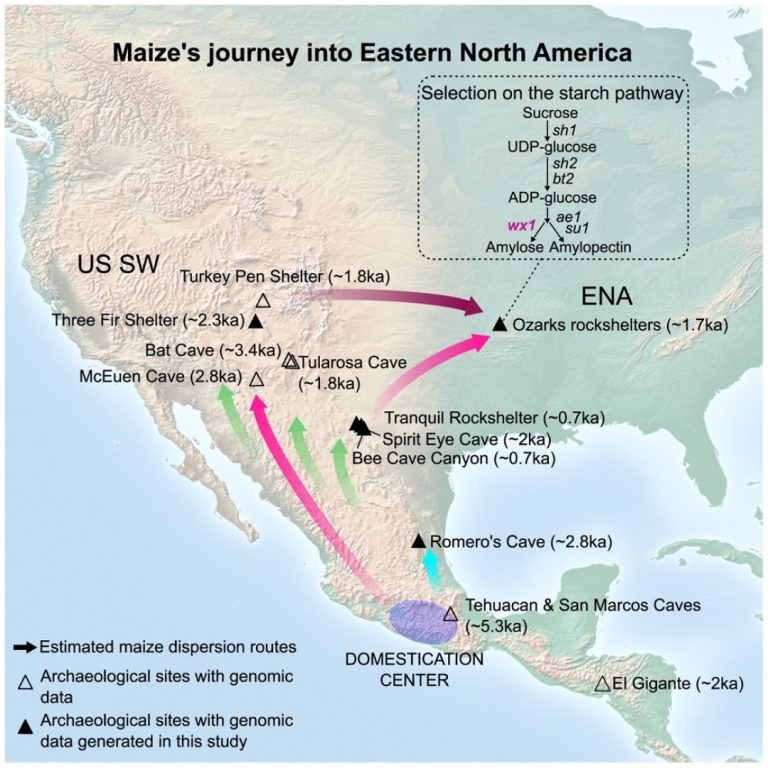
On Dec. 4, 2024, an international team of scientists led by the Center for Evolutionary Hologenomics, GLOBE Institute,…