H5N1 strikes more U.S. poultry flocks, pet cats
On Jan. 22, 2025, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) has…

On Jan. 22, 2025, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) has…

On Jan. 22, 2025, researchers have identified chronic wasting disease (CWD) prions in raw, cooked, and cured meat…
On Jan. 21, 2025, researchers from the University of Alberta announced they have come up with a way…

On Jan. 21, 2025, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) approval of a…

On Jan. 20, 2025, the White House announced the United States will exit the World Health Organization (WHO)….
On Jan. 21, 2025, Rice University announced that four research groups are part of an inaugural cohort of…
On Jan. 20, 2025, the World Health Organization (WHO) announced that Tanzania has confirmed an outbreak of Marburg…

On Jan. 20, 2025, an analysis of data from nearly 2 million people has revealed new insights into…
On Jan. 20, 2025, a large international 68-country survey of 71,922 respondents found that in most countries, most…

On Jan. 17, 2025, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) reported that an…
On Jan. 17, 2025, The U.S. government announced it had awarded Moderna $590 million to advance the development…

On Jan. 17, 2025, the U.S. Centers for Disease Control and Prevention (CDC) reported that eleven pediatric deaths…

On Jan. 16, 2025, Colossal Biosciences – the company behind an ambitious, Jurassic Park-style effort to revive extinct bird and…

On Jan. 16, 2025, Shionogi Inc. has been awarded a $375 million project agreement through the Rapid Response…

On Jan. 15, 2025, officials from Lincoln Park Zoo in Chicago reported that testing had confirmed the highly pathogenic…
On Jan. 15, 2025, the U.S. Department of Commerce’s Bureau of Industry and Security (BIS) released an Interim…

On Jan. 15, 2025, the California Department of Public Health (CDPH) reported thatqOne confirmed human infection with influenza…
On Jan. 14, 2025, The U.S. Department of Defense (DoD) has awarded up to $11.9 million to Oregon…

On Jan. 14, 2025, the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) announced…

On Jan. 14, 2025, an international research team led by the University of Edinburgh reported new genetic risk…

On Jan. 13, 2025, the Texas Animal Health Commission (TAHC) and the United States Department of Agriculture’s (USDA)…
On Jan. 13, 2025, a study led by NYU Langone Health shows that the risk of developing dementia at…

On Jan. 13, 2025, new research identifies differing trends in attention-deficit/hyperactivity disorder (ADHD) diagnoses among adolescents and adults,…

On Jan. 13, 2025, the Los Angeles County Department of Public Health (LACDPH) reported that five indoor-only cats…

On Jan. 13, 2025, the World Health Organization (WHO) informed its Member States and IHR State Parties of…
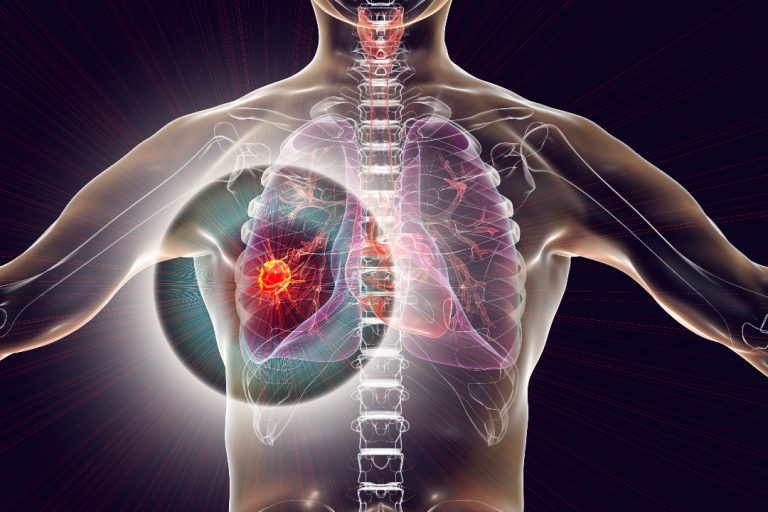
On Jan. 13, 2025, scientists from the Francis Crick Institute, UCL, UCLH and Personalis announced they have found…

On Jan. 10, 2025, the Institute for Health Metrics and Evaluation (IHME) reported that when it comes to…

On Jan. 10, 2025, a study of chronic wasting disease (CDW), the prion disease that causes deer, elk…
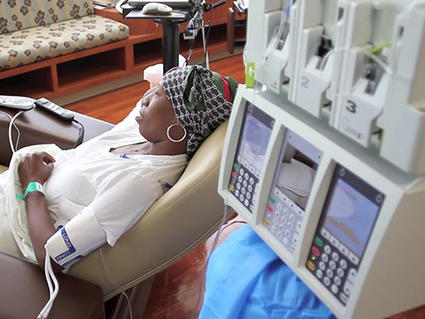
On Jan. 10, 2025, researchers at Roswell Park Comprehensive Cancer Center have identified a previously unknown cause of…
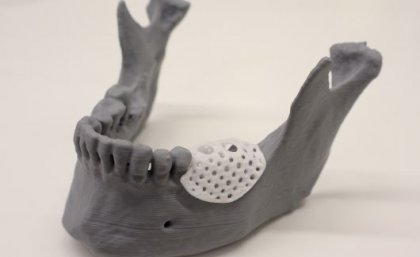
On Jan. 9, 2025, clinicians from the The University of Queensland (UQ) announced they had successfully used custom-made…