Researchers unlock genetic code of cancer-causing liver fluke parasite
On Sept. 10, 2014, an international team of scientists from Singapore, Thailand, China and Australia announced that they…

On Sept. 10, 2014, an international team of scientists from Singapore, Thailand, China and Australia announced that they…

On Sept. 8, 2014, the University of Washington and UW Medicine announced that Mary-Claire King, UW professor of…

On Sept. 5, 2014, researchers led by Professor Ilkka Hanski at the University of Helsinki announced they had…

On Sept. 3, 2014, Oregon Health & Science University (OHSU) announced it had been awarded a $25 million…

On Aug. 21, 2014, Mark McAndrew, a resident of McKinney, Texas, gave $2.4 million to the University of…
On Aug. 14. 2014, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Avastin (bevacizumab)…

On Aug. 5, 2014, a team of scientists from around the world led by Baylor College of Medicine…

On Aug. 5, 2014, the genome sequence of the common cat was published in GigaScience. The DNA of…

On Aug. 4, 2014, the Allen Institute for Brain Science, California Institute of Technology, New York University School…

On Jul. 29, 2014, Amgen announced closure of former Boulder-based Synergen LakeCentre facility. Amgen acquired Boulder-based Synergen in…

On Jul. 29, 2014, Amgen announced the closure of facilities in Seattle and Bothell which resulted in the…

On Jul. 28, 2014, OHSU announced it has received a $100 million gift from an unnamed donor who…

On Jul. 22, 2014, The Broad Institute announced an unprecedented commitment of $650 million from philanthropist Ted Stanley…

On Jul. 19, 2014, the U.S. Centers for Disease Control and Prevention (CDC) responded to a multi-state outbreak…

On Jul. 17, 2014, the International Wheat Genome Sequencing Consortium (IWGSC) a draft sequence of the bread wheat…

On Jul. 10, 2014, an international confederation of biotechnology trade associations ratified bylaws that created the International Council…

On Jul. 9, 2014, the U.S. Centers for Disease Control and Prevention (CDC) activated its Emergency Operations Center…

On Jul. 7, 2014, Cold Spring Harbor Laboratory (CSHL) announced a $50 million gift from Jim and Marilyn…

On Jul. 1, 2014, Genentech entered into an agreement to acquire Seragon Pharmaceuticals, a privately held biotechnology company…

On Jun. 30, 2014, leaders from Case Western Reserve University and University Hospitals announced that Char and Chuck…

On Jun. 26, 2014, a $300 million building opened in Portland is shared by Portland State University and Oregon…

On Jun. 20, 2014, the U.S. Centers for Disease Control and Prevention (CDC) published Immunization Practices Advisory Committee…

On Jun. 17, 2014 the University of Calgary received $200 million from International businessman and University of Calgary…

On Jun. 2, 2014, Omeros received U.S. Food and Drug Administration (FDA) approval of Omidria for use in…
On May 20, 2014, Keck Medicine of USC received a $2 million gift from The Eli and Edy…
On May 7, 2014, scientists at The Scripps Research Institute (TSRI) announced that they had engineered a bacterium…
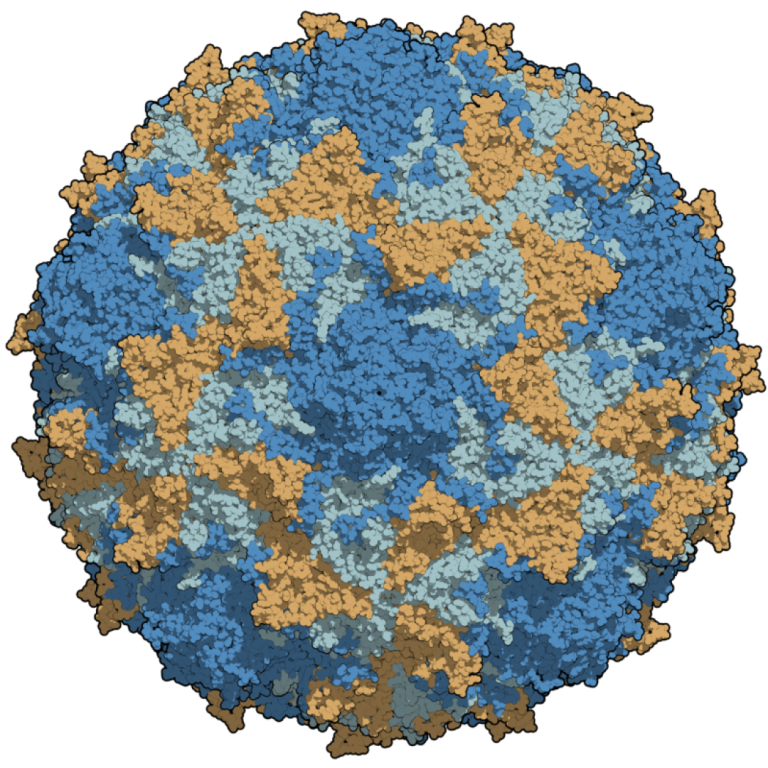
On May 5, 2014, the World Health Organization (WHO) Director-General declared the international spread of wild poliovirus a…
On May 1, 2014, the first U.S. imported case of Middle East Respiratory Syndrome (MERS) was confirmed in…

On Apr. 24, 2014 an international team of scientists announced they had deciphered the genetic code of the…

On Apr. 14, 2014, Stanford Medicine researchers announced a study that found a gene variant puts women at…