Trevena announced results of TRV027 proof-of-concept study in COVID-19 patients
On Sept. 30, 2021, Trevena announced data from 30 patients enrolled in the proof-of-concept study of TRV027, the…

On Sept. 30, 2021, Trevena announced data from 30 patients enrolled in the proof-of-concept study of TRV027, the…
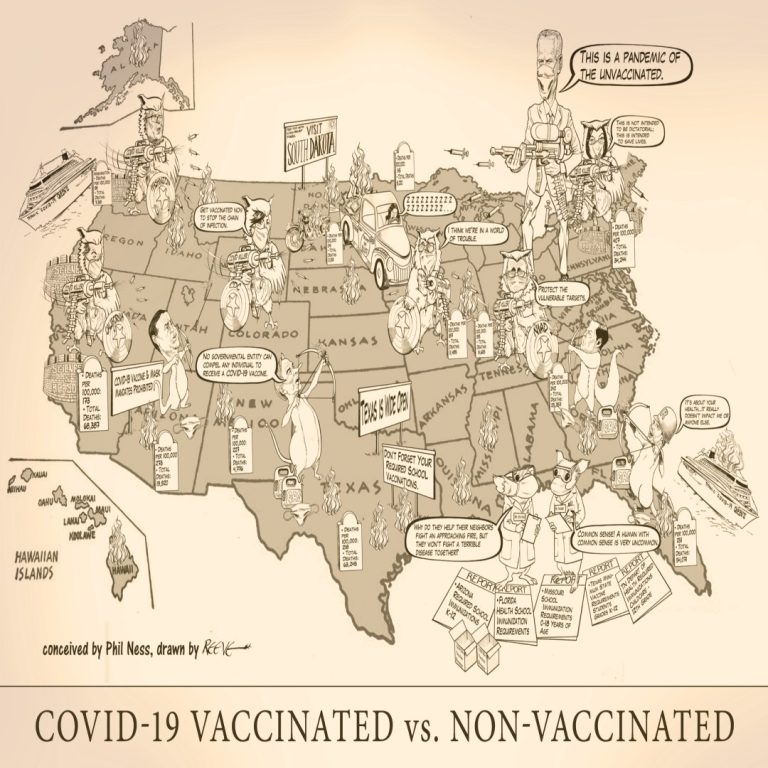
This cartoon illustrates the various issues facing individuals across the U.S. who are vaccinated against COVID-19 verus those…

On Sept. 29, 2021, Colossal, a de-extinction company that is combining the science of genetics with the business…
On Sept. 29, 2021, Regeneron announced that the New England Journal of Medicine (NEJM) published positive detailed results…

On Sept. 29, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 29, 2021, Roche confirmed positive data from the phase II/III 2066 study, investigating Ronapreveル (casirivimab and…

On Sept. 29, 2021, the University of Pennsylvania announced that the Breakthrough Prize in Life Sciences was awarded…

On Sept. 29, 2021, VBI Vaccines announced positive results from multiple preclinical studies against several COVID-19 variants of…

On Sept. 29, 2021, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On Sept. 28, 2021, the University of Missouri researcher Kamlendra Singh led a team that analyzed protein sequences…

On Sept. 28, 2021, AIM ImmunoTech announced that is had submitted a Pre-Investigational New Drug application (Pre-IND) to…

On Sept. 28, 2021, Sanofi announced that it had terminated plans for its own mRNA-based COVID-19 vaccine given…

On Sept. 28, 2021, Quest Diagnostics reported that one in two (50.5%) American children under six years of…
On Sept. 28, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 28, 2021, Soligenix announced publication of pre-clinical immunogenicity studies for CiVax (heat stable COVID-19 vaccine program)…

On Sept. 28, 2021, TGen, an affiliate of City of Hope, will use its MindCrowd website portal to…
On Sept. 28, 2021, the National Institute of Allergy and Infectious Diseases announced it had awarded approximately $36.3…

On Sept. 27, 2021, Pfizer announced the start of the Phase 2/3 EPIC-PEP (Evaluation of Protease Inhibition for…

On Sept. 27, 2021, RELIEF THERAPEUTICS reported its U.S. collaboration partner, NRx Pharmaceuticals, had announced top line data…

On Sept. 27, 2021, Pfizer announced that the first participants have been dosed in a Phase 1 clinical…

On Sept. 24, 2021, Moderna announced a supply agreement with the government of Peru for 20 million doses…

On Sept. 24, 2021, the U.S. Department of Agriculture’s (USDA) announced confirmation of SARS-CoV-2 in a ferret in…

On Sept. 23, 2021, Twist Bioscience reported that its internally-discovered antibody candidate TB202-3 (CoVIC-094), demonstrated potent binding to…

On Sept. 23, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) has authorized…

On Sept. 23, 2021, Novavax announced publication of complete results from a pivotal Phase 3 clinical trial of…

On Sept. 23, 2021, Novavax with its partner, Serum Institute of India announced a regulatory submission to the…
On Sept. 23, 2021, OraSure Technologies and the Biomedical Advanced Research Development Authority (BARDA) announced up to $13.6…

On Sept. 23, 2021, Tonix Pharmaceuticals announced it had expanded its research collaboration with Columbia University. The research…

On Sept. 22, 2021, Inovio Pharmaceuticals announced that it had received authorization from COFEPRIS, the national health regulatory…

On Sept. 22, 2021, Pfizer and BioNTech announced plans to expand their agreement with the U.S. government by…