Moderna completed application to U.S. FDA for EUA of omicron-targeting bivalent Covid-19 booster vaccine
On Aug. 22, 2022, the Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of Moderna…

On Aug. 22, 2022, the Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of Moderna…
On Aug. 19, 2022, Novavax announced that the Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received expanded emergency use…

On Aug. 18, 2022, Novavax announced that New Zealand’s Medsafe had granted expanded provisional approval for Nuvaxovid (NVX-CoV2373)…
On Aug. 18, 2022, the White House announced that the HHS had accelerated Phase 4 of its National…

On Aug. 17, 2022, the U.S. Food and Drug Administration approved Zynteglo (betibeglogene autotemcel) developed by bluebird bio,…

On Aug. 16, 2022, UNICEF announced that it had awarded a contract for the first ever supply of…

On Aug. 15, 2022, Moderna announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK…
On Aug. 15, 2022, the U.S. Department of Health and Human Services announced that was making up to…

On Aug. 15, 2022, Roche announced the launch of the Elecsysᆴ IGRA SARS-CoV-2 test in countries that accept…
On Aug. 12, 2022, a group of global experts convened by WHO agreed on new names for monkeypox…

On Aug. 12, 2022, Novavax announced that partner, SK bioscience, had received a Post Approval Change Application approval…

On Aug. 11, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a supplemental…

On Aug. 9, 2022, the the Department of Health and Human Services (HHS) announced it had determined that…
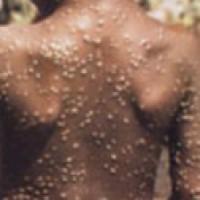
On Aug. 9, 2022, the U.S. Food and Drug Administration issued an emergency use authorization for the JYNNEOS…

On Aug. 9, 2022, Moderna announced an amendment to its agreement with the European Commission (EC) to convert…

On Aug. 5, 2022, the Centers for Disease Control and Prevention (CDC) reported the first human infection with…

On Aug. 5, 2022, researchers from the National Institutes of Health announced that they had developed a three-dimensional…
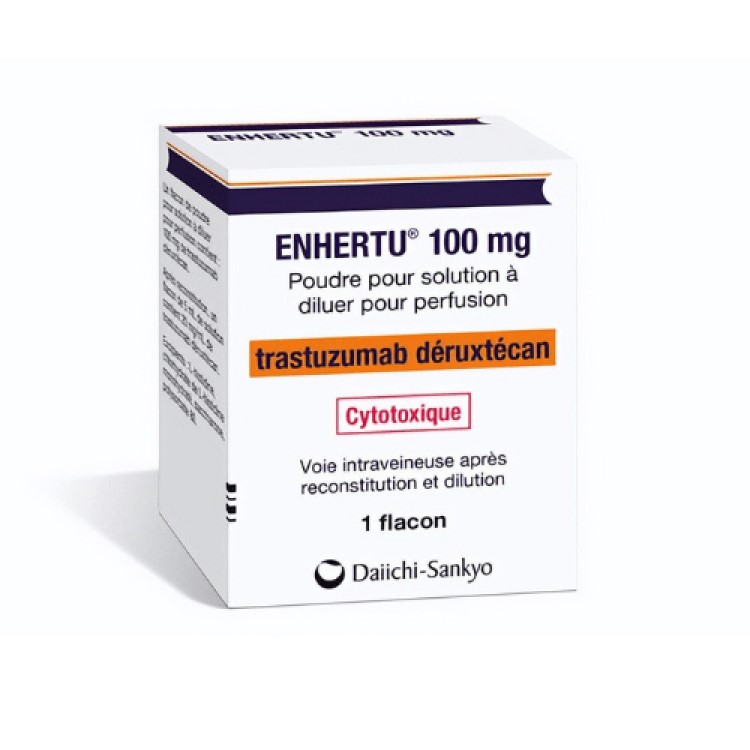
On Aug. 5, 2022, the U.S. Food and Drug Administration (FDA) approved Daiichi Sankyo’s Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV…
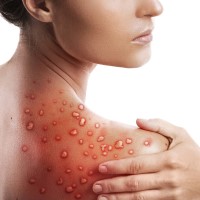
On Aug. 4, 2022, the U.S. Department of Health and Human Services declared the ongoing spread of monkeypox…

On Aug. 4, 2022, Novavax announced the initiation of its Phase 2b/3 Hummingbird global clinical trial. The trial…

On Jul. 31, 2022, the National Aeronautics and Space Administration (NASA) paid respects to actor Nichelle Nichols, who…

On Jul. 30, 2022, scientists announced they had sequenced the genome of a 6,000-year-old Citrullus seeds that revealed…

On Jul. 29, 2022, the U.S. Department of Health and Human Services (HHS), in collaboration with the U.S….

On Jul. 28, 2022, AlphaFold, in partnership with EMBL’s European Bioinformatics Institute (EMBL-EBI), announced they we’re releasing predicted…
On Jul. 28, 2022, the World Health Organization (WHO) released new guidelines for the use of long-acting injectable…
On Jul. 28, 2022, the Allen Institute announced a newly released dataset of 1.2M cells from brain donors…

On Jul. 27, 2022, BD (Becton, Dickinson) announced their newly developed molecular polymerase chain reaction (PCR) test for…

On Jul. 27, 2022, the Centers for Disease Control and Prevention (CDC) announced that it had identified for…

On Jul. 27, 2022, Pfizer and BioNTech announced that the companies had initiated a randomized, active-controlled, observer-blind, Phase…
On Jul. 27, 2022, City of Hope announced that a 66-year-old man who was diagnosed with HIV in…