Novavax Nuvaxovid COVID-19 vaccine became available in Israel for individuals aged 12 and older
On Sept. 16, 2022, Novavax announced that the Israel Ministry of Health had granted an import and use…

On Sept. 16, 2022, Novavax announced that the Israel Ministry of Health had granted an import and use…

On Sept. 15, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Sept. 15, 2022, the National Institutes of Health announced that a research team funded by the NIH…
On Sept. 15, 2022, Gilead Sciences announced updates to the World Health Organizationメs Therapeutics and COVID-19: living guideline,…
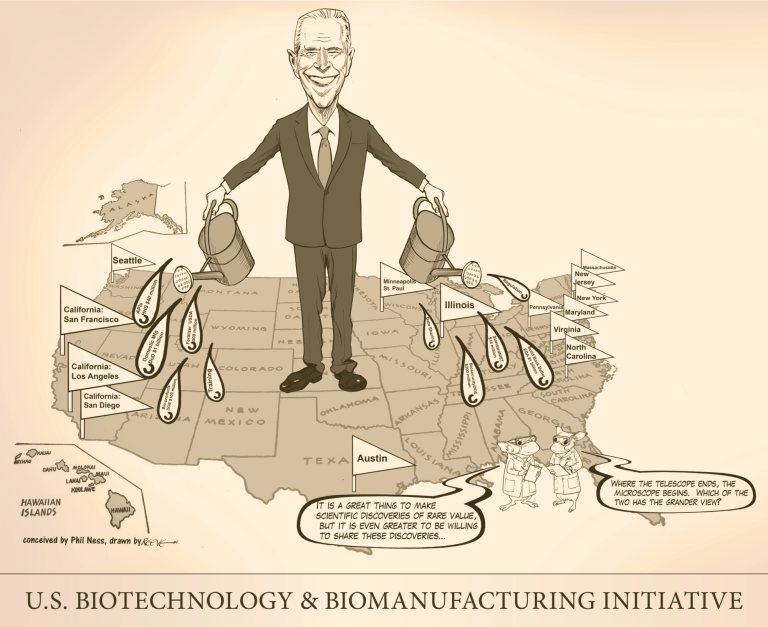
On Sept. 14, 2022, The U.S. Biotech Initiative was announced detailing new investments and resources to advance the…
On Sept. 13, 2022, Novavax and Serum Institute of India, the world’s largest vaccine manufacturer by volume, announced…

On Sept. 13, 2022, the National Institutes of Health announced it will invest $130 million over four years,…

On Sept. 12, 2022, the White House announced an executive order to advance biotechnology and biomanufacturing towards innovative…
On Sept. 8, 2022, a clinical trial evaluating alternative strategies for administering the JYNNEOS mpox vaccine to increase…

On Sept. 8, 2022, researchers from the University of Oxford and their partners reported findings from their Phase…

On Sept. 8, 2022, the U.S. Department of Agricultureメs Animal and Plant Health Inspection Service launched an updated…

On Sept. 8, 2022, the Fred Hutchinson Cancer Center (FHCRC) announced that Stuart and Molly Sloan had pledged…

On Sept. 7, 2022, the U.S. Food and Drug Administration (FDA) announced it had approved Revance Therapeutics’ DAXXIFY…

On Sept. 7, 2022, Quest Diagnostics announced that it had received emergency use authorization (EUA) from the U.S….
On Sept. 6, 2022, scientists in China announced that they had conducted the first comprehensive study of the…

On Sept. 6, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service had reviewed…

On Sept. 2, 2022, Novavax announced that Swissmedic, the Swiss Agency for Therapeutic Products, had expanded its temporary…

On Sept. 2, 2022, Novavax announced that the World Health Organization (WHO) had approved a variation to allow…

On Sept. 1, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had been recommended for expanded conditional marketing…

On Aug. 31, 2022, a new front-door to the University of Victoria opened to advance health and life…

On Aug. 31, 2022, the Yale New Haven Hospital broke ground on the $838 million, 505,000 square foot…

On Aug. 29, 2022, a group of international researchers, coordinated by Dr Jo�o Teixeira at The Australian National…

On Aug. 29, 2022, the U.S. Department of Health and Human Services (HHS) announced it had provided approximately…

On Aug. 26, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Aug. 26, 2022, Pfizer and BioNTech announced they had completed a submission to the European Medicines Agency…
On Aug. 24, 2022, BioMarin Pharmaceutical announced that the European Commission (EC) had granted conditional marketing authorization (CMA)…

On Aug. 23, 2022, Health authorities in the Democratic Republic of the Congo declared a resurgence of Ebola…

On Aug. 23, 2022, Pfizer and BioNTech announced updated efficacy results from a Phase 2/3 trial evaluating a…

On Aug. 22, 2022, the Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of Moderna…

On Aug. 23, 2022, Moderna announced that the Government of Canada had exercised its option to purchase an…