Scientists revealed encouraging findings in first-in-human clinical trial evaluating HIV vaccine approach
On Dec. 2, 2022, in a multi-center collaboration led by Scripps Research, scientists announced they have achieved promising…

On Dec. 2, 2022, in a multi-center collaboration led by Scripps Research, scientists announced they have achieved promising…

On Dec. 2, 2022, the Oregon Department of Agriculture (ODA) and the U.S. Department of Agriculture’s (USDA) Animal…

On Nov. 29, 2022, Novavax announced that the World Health Organization had issued an updated Emergency Use Listing…

On Nov. 28, 2022, the U.S. Department of Agricultureメs Research Service, Auburn University’s College of Agriculture, and the…
On Nov. 28, 2022, the WHO announced that following a series of consultations with global experts, it had…

On Nov. 27, 2022, it was reported a parasite that infects up to one-third of people around the…

On Nov. 24, 2022, researchers Kanta Horie and Chihiro Sato at the Tracy Family SILQ Center at Washington…

On Nov. 22, 2022, the U.S. Food and Drug Administration approved the investigational gene therapy etranacogene dezaparvovec or…

On Nov. 21, 2022, the WHO is launching a global scientific process to update the list of priority…

On Nov. 21, 2022, the National Institutes of Health awarded more than $12 million to three institutions for…

On Nov. 18, 2022, Pfizer and BioNTech announced results from an analysis examining the immune response induced by…

On Nov. 18, 2022, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid (COVID-19 Vaccine (Recombinant…

On Nov. 18, 2022, the Journal of the American Medical Association reported that the U.S. experienced high COVID-19…

On Nov. 18, 2022, the World Health Organisation for Animal Health (WHO) announced that Peru had reported its…

On Nov. 17, 2022, the U.S. Food and Drug Administration (FDA) approved Tzield (teplizumab-mzwv) injection to delay the…

On Nov. 16, 2022, Pfizer and BioNTech announced that the companies had initiated a Phase 1 study to…

On Nov. 16, 2022, the Cancer Prevention and Research Institute of Texas (CPRIT) approved $12 million in recruitment…

On Nov. 16, 2022, researchers at the National Institutes of Health (NIH) announced they had successfully identified differences…

On Nov. 15, 2022, Roche announced that the U.S. Food and Drug Administration had granted Emergency Use Authorization…

On Nov. 15, 2022, Moderna reported findings from a Phase II/III clinical trial where bivalent Omicron-targeting booster candidates,…

On Nov. 15, 2022, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Nov. 15, 2022, the U.S. Food and Drug Administration conditionally approved Panoquell-CA1 (fuzapladib sodium for injection) for…
On Nov. 14, 2022, in a study published in Nature Communications, researchers discovered the pathway by which chronic…

On Nov. 10, 2022, Pfizer and BioNTech announced a booster dose of their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine…

On Nov. 10, 2022, researchers from the Okinawa Institute of Science and Technology (OIST), in collaboration with K….
On Nov. 9, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Nov. 9, 2022, researchers at the University of Missouri announced they had identified the highly prevalent, specific…
On Nov. 8, 2022, Novavax announced topline results from its Phase 3 Boosting Trial for the SARS-CoV-2 rS…
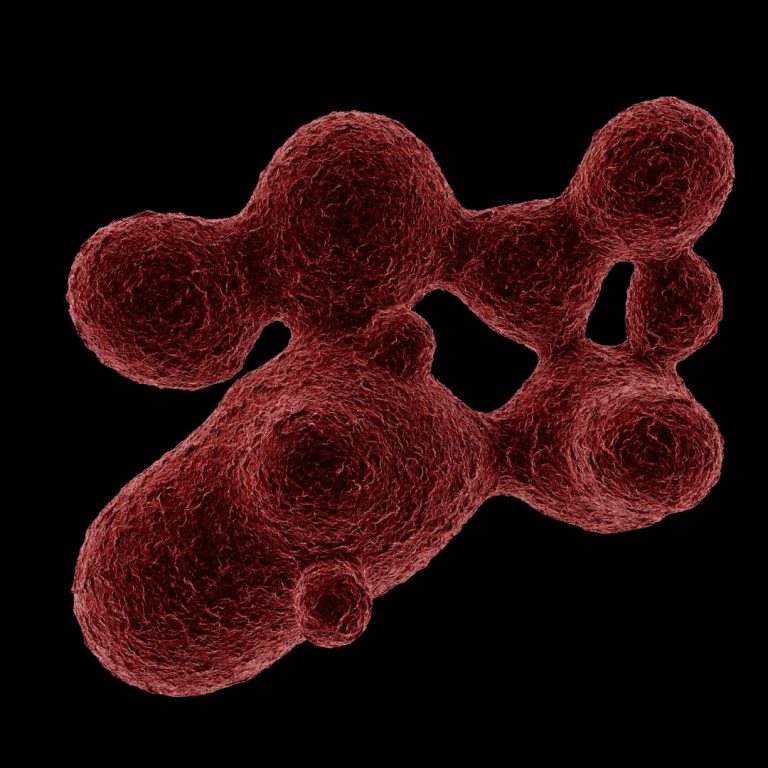
On Nov. 7, 2022, the NHS Blood and Transplant (NHSBT) reported that red blood cells grown in a…

On Nov. 5, 2022, the U.S. Department of Agriculture’s (USDA) Animal Plant Health Inspection Service (APHIS) confirmed the…