CDC reported two human infections with swine flu viruses
On Aug. 4, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported the first two U.S….

On Aug. 4, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported the first two U.S….

On Aug. 4, 2023, Biogen and Sage Therapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…

On Aug. 1, 2023, the U.S. Dept of Agriculture (USDA) announced the groundbreaking of a new Plant Sciences…

On Aug. 1, 2023, BD announced that the U.S. Food and Drug Administration (FDA) 510(k) had provided clearance…
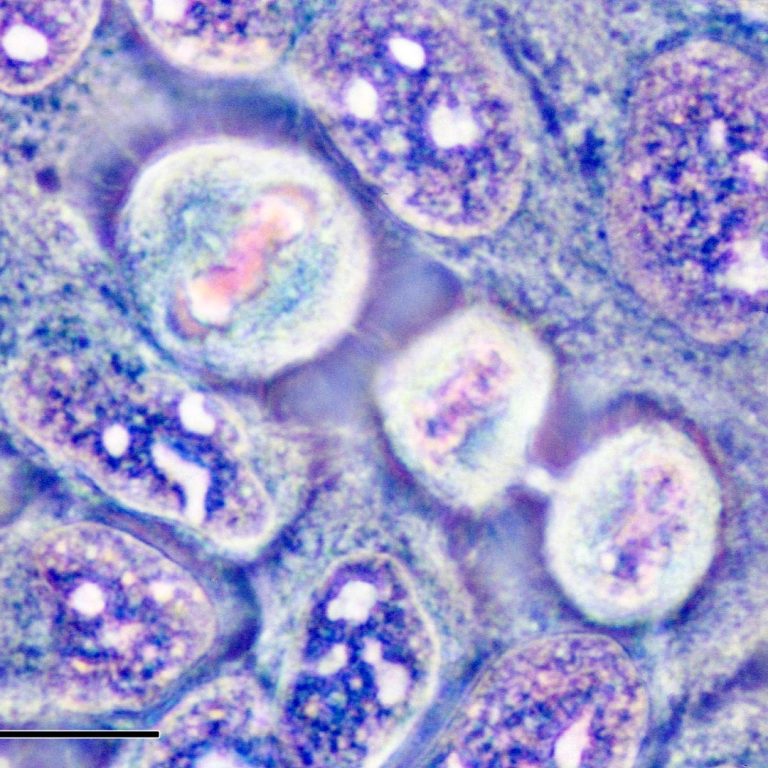
On Aug. 1, 2023, more than 70 years after doctors at Johns Hopkins Hospital took Henrietta Lacks’ cervical…

On Aug. 1, 2023, the Finnish Food Authority specified the euthanasia criteria for fur animals infected with avian…
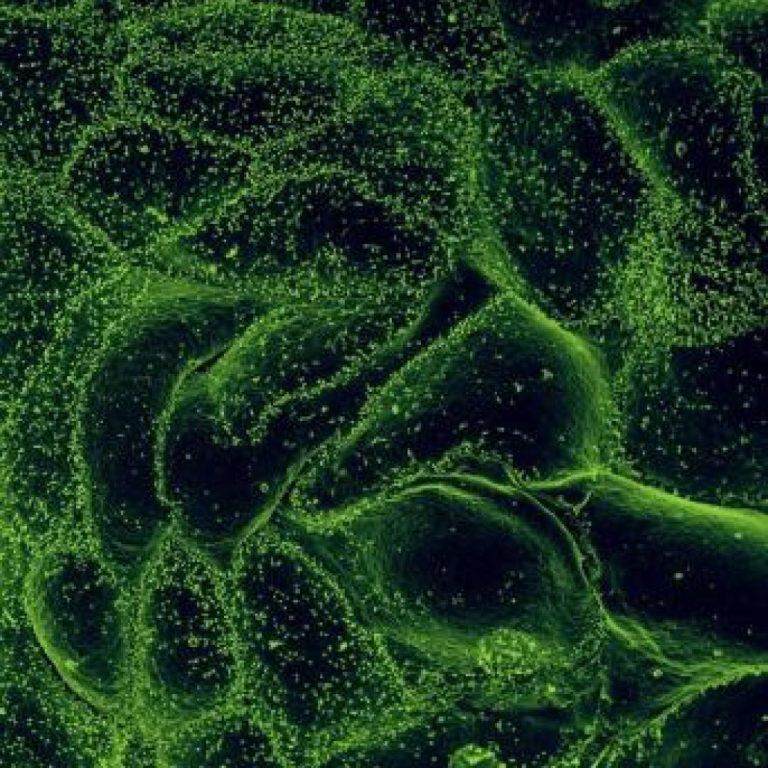
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
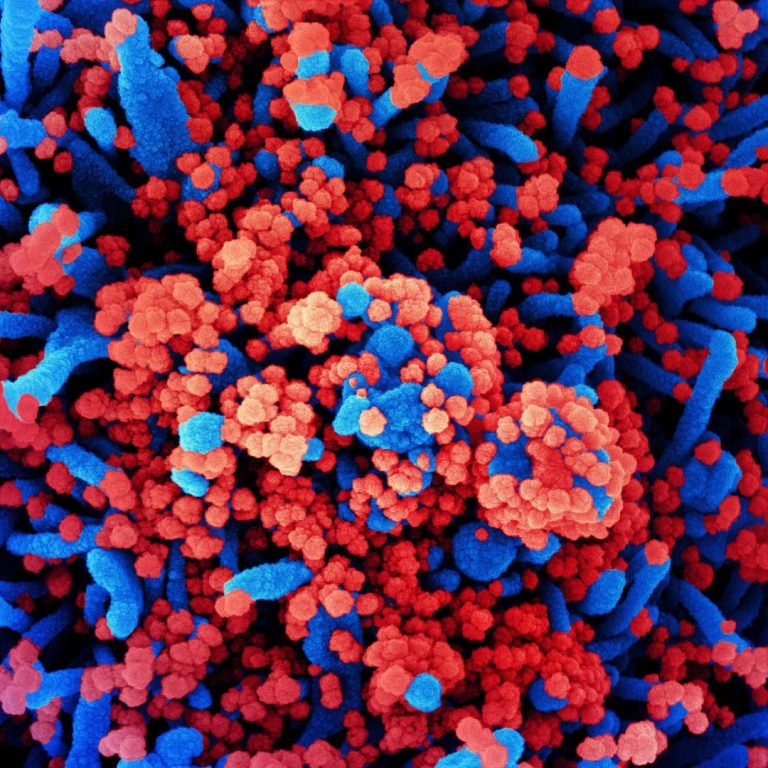
On Jul. 31, 2023, scientists at Washington University in St. Louis have developed a breath test that quickly…

On Jul. 31, 2023, the U.S. Department of Health and Human Services announced the formation of the Office…
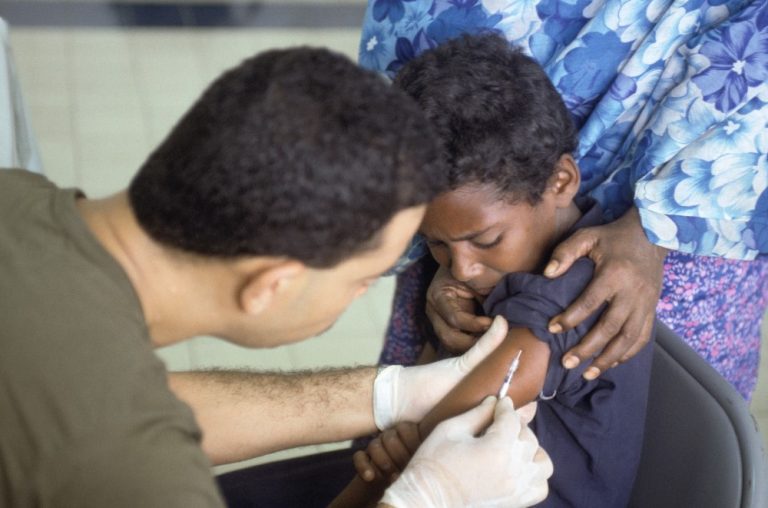
On Jul. 28, 2023, the World Health Organization (WHO) reported circulating vaccine-derived poliovirus type 2 (cVDPV2) in the…
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…
On Jul. 28, 2023, COVID vaccine uptake among hospital workers at a Philadelphia teaching hospital rose from 86%…
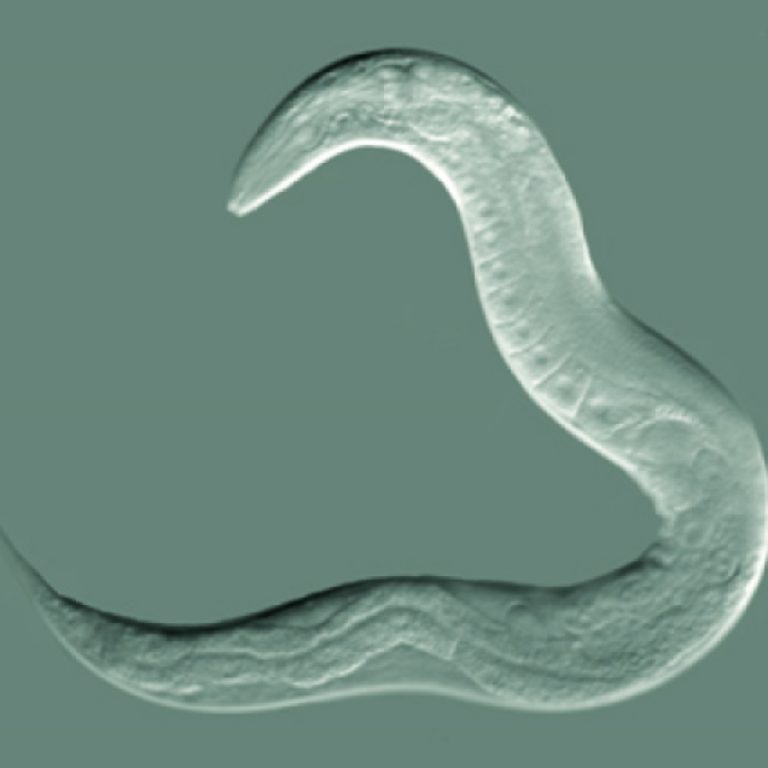
On Jul. 28, 2023, researchers from the Institute of Zoology at the University of Cologne, the Max Planck…
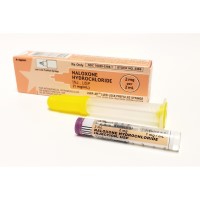
On Jul. 28, 2023, the U.S. FDA approved RiVive, 3 milligram (mg) naloxone hydrochloride nasal spray for over-the-counter…

On Jul. 28, 2023, in a first-of-its-kind clinical trial, bioelectronic medicine researchers, engineers and surgeons at Northwell Healthメs…

On Jul. 27, 2023, the Centers for Disease Control and Prevention (CDC) reported that between 2010 and 2022,…

On Jul. 27, 2023, SIGA Technologies announced that the U.S. Department of Health and Human Services exercised procurement…

On Jul. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $50.1 million to fund clinical-stage research…

On Jul. 27, 2023, the IHR National Focal Point of Poland notified WHO of unusual deaths in cats…

On Jul. 25, 2023, U.S. National Science Foundation-supported research, led by Joshua Rosenthal of the Marine Biological Laboratory…
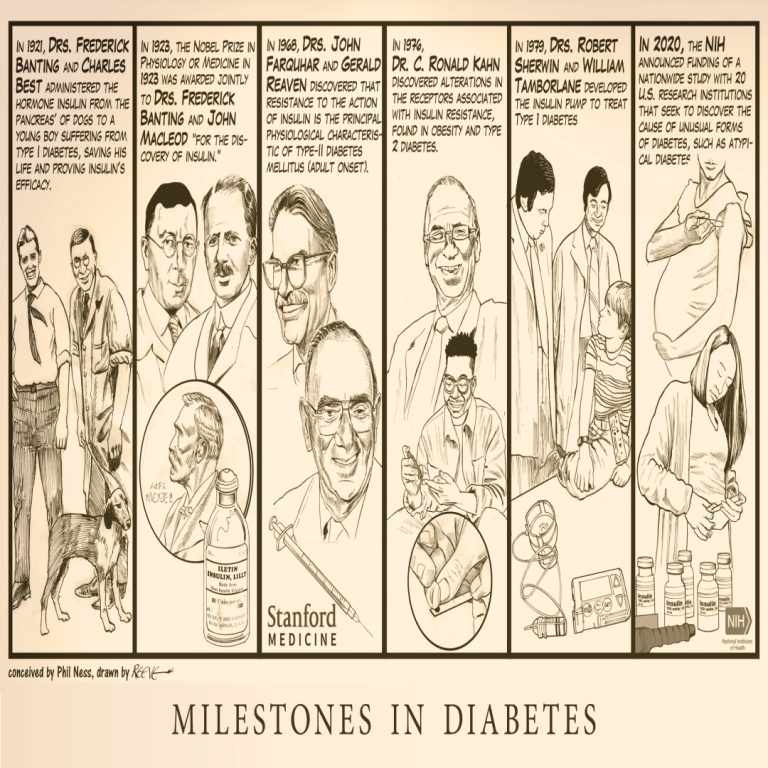
Milestones in Diabetes illustrates major milestones from the first administration of the hormone insulin in 1921 to a…
On Jul. 24, 2023, the United Arab Emirates (UAE), notified WHO of a case of Middle East Respiratory…

On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…

On Jul. 20, 2023, Emergent BioSolutions announced that the U.S. Food and Drug Administration (FDA) had approved CYFENDUSル…

On Jul. 20, 2023, seven innovative pharmaceutical companies announced they had established the INTREPID Alliance, with the goal…

On Jul. 19, 2023, a team of scientists from the Translational Genomics Research Institute (TGen), part of the…

On Jul. 19, 2023, the National Institute of Health announced that a nationwide research team had created the…
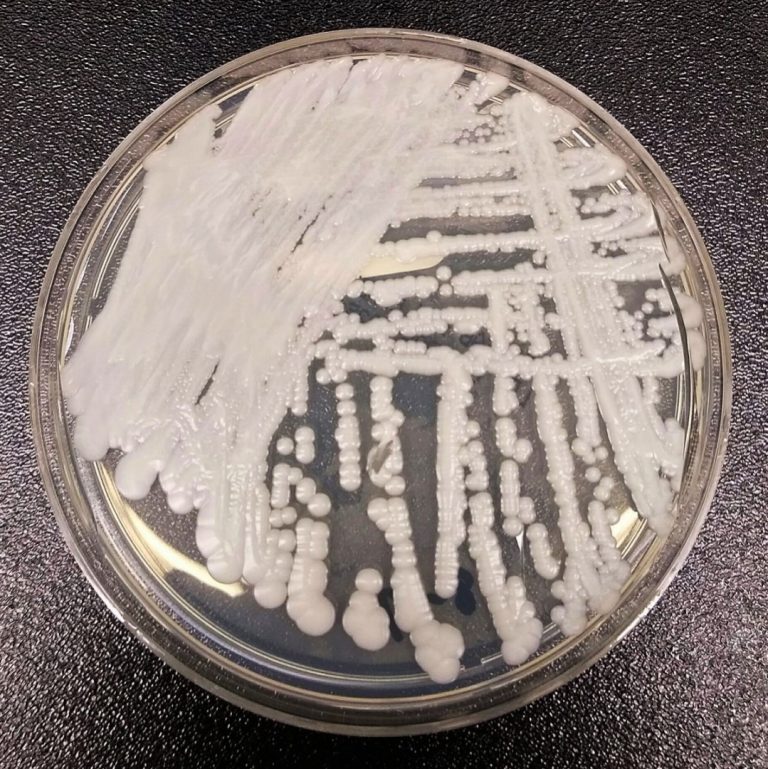
On Jul. 19, 2023, the King County Department of Health issued a Health Advisory that Candida auris (C….

On Jul. 18, 2023, scientists reported that they had extracted, sequenced, and analyzed historical RNA from muscle and…