COVID-19 vaccinations shifted to regular immunization as COVAX draws to a close
On Dec. 19, 2023, COVAX, the multilateral mechanism for equitable global access to COVID-19 vaccines launched in 2020,…

On Dec. 19, 2023, COVAX, the multilateral mechanism for equitable global access to COVID-19 vaccines launched in 2020,…

On Dec. 18, 2023, the Novo Nordisk Foundation announced it was committing up to DKK 1.8 billion (USD…

On Dec. 18, 2023, W. Kimryn Rathmell, M.D., Ph.D., began work as the 17th director of the National…

On Dec. 18, 2023, Novavax announced that the Taiwan Food and Drug Administration had granted emergency use authorization…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…

On Dec. 18, 2023, COVID-19 vaccine maker BioNTech (22UAy.DE) announced that it aims to start production at its…

On Dec. 15, 2023, In a pivotal move towards addressing one of the worldメs most underrecognized health challenges,…

On Dec. 14, 2023, the University of Alberta announced that a study published in Nature sheds light on…

On Dec. 14, 2023, The Coalition for Epidemic Preparedness Innovations (CEPI) and PATH today announced a renewed collaboration…

On Dec. 14, 2023, the Department of Energy’s (DOE) Pacific Northwest National Laboratory (PNNL) announced that it had…

On Dec. 14, 2023, Biogen and Sage Therapeutics announced ZURZUVAE (zuranolone) 50 mg (two 25 mg capsules per…

On Dec. 14, 2023, Pfizer announced it had completed the acquisition of Seagen, a global biotechnology company based…
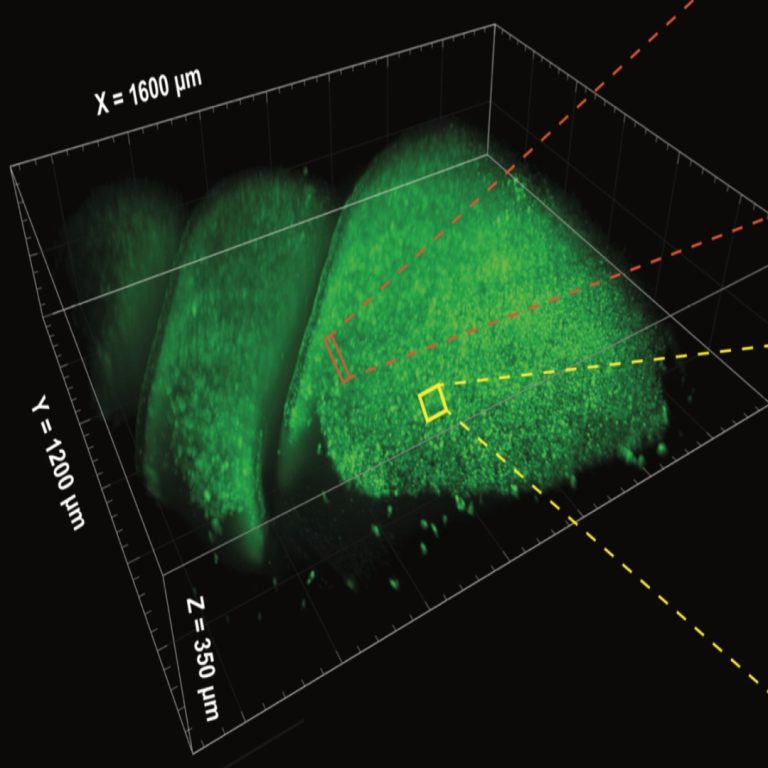
On Dec. 13, 2023, for the first time ever, an international team of researchers announced they had created…

On Dec. 13, 2023, in a study of historic scale, scientists at Gladstone Institutes announced that they had…

On Dec. 13, 2023, National Institute of Allergy and Infectious Diseases (NIAID) scientists announced findings published in the…
On Dec. 13, 2023, the Allen Institute and collaborators announced that after six years and 32 million cells,…
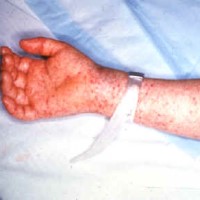
On Dec. 11, 2023, the U.S. Centers for Disease Control and Prevention (CDC) issued a Health Alert Network…

On Dec. 8, 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Dec. 8, 2023, the University of Virginia broke ground Friday on the Paul and Diane Manning Institute…
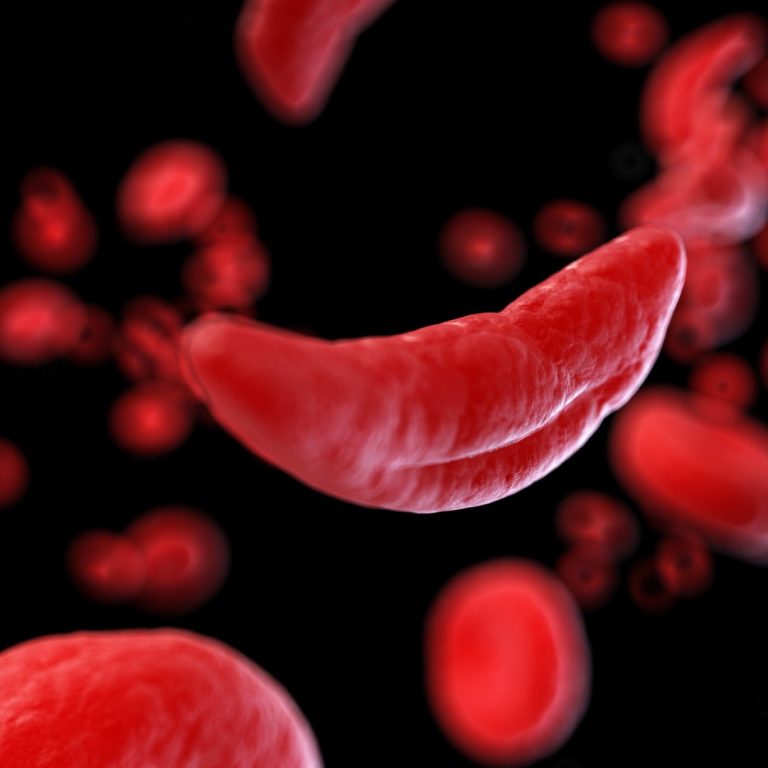
On Dec. 8, 2023, the U.S. Food and Drug Administration (FDA) approved two milestone treatments, Casgevy and Lyfgenia,…

On Dec. 8, 2023, bluebird bio announced the U.S. commercial launch of its LYFGENIA’ (lovotibeglogene autotemcel, also known…

On Dec. 7, 2023, BioNTech announced that it had signed a multi-year strategic partnership with the State of…

On Dec. 7, 2023, the U.S. Food and Drug Administration reported that of the more than 116 medically…
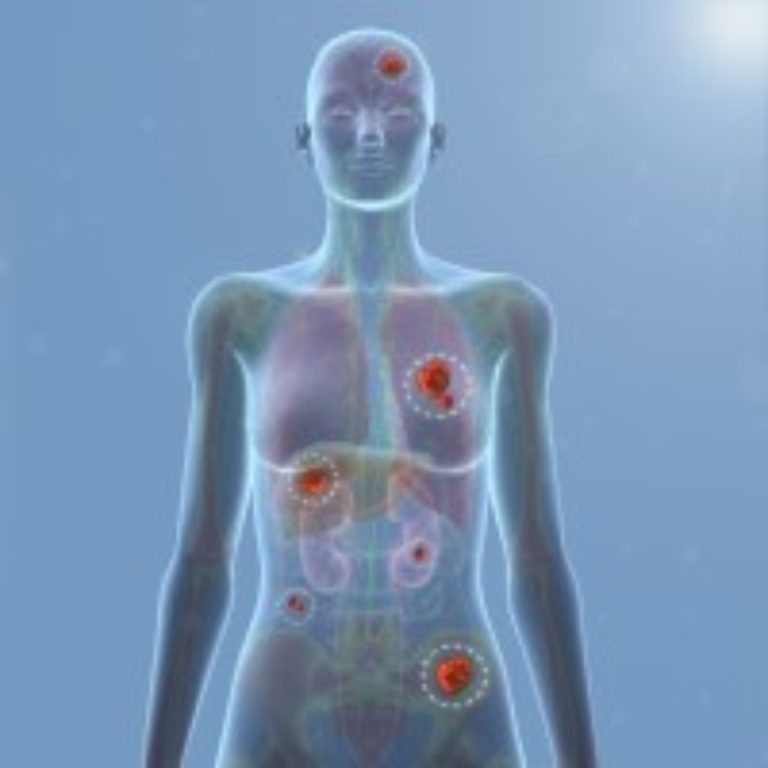
On Dec. 6, 2023, Anixa Biosciences announced updated positive results from the Phase 1 clinical trial of its…
On Dec. 6, 2023, the University of Missouri Research Reactor (MURR) announced they had completed its first commercial…

On Dec. 6, 2023, the federal government announced it had expanded the Home Test to Treat program, an…

On Dec. 5, 2023, Novavax announced that Health Canada had granted expanded authorization for Nuvaxovid XBB.1.5 Vaccine (Recombinant…
On Dec. 5, 2023, Novartis announced that the U.S. Food and Drug Administration (FDA) had approved Fabhalta (iptacopan)…

On Dec. 4 2023, the University of Wisconsin-Madison researchers announced that while analyzing data from wastewater samples collected…

On Dec. 3, 2023, the Bill & Melinda Gates Foundation announced that global donors at COP28 had pledged…