Vietnam reported first human infection with avian influenza H9N2 virus
On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…

On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…
On Apr. 12, 2024, Oregon Health Sciences University (OHSU) announced research that shows using live SARS-CoV-2 virus revealed…

On Apr. 11, 2024, a study of data from more than 2 million children in Sweden reported that…

On Apr. 10, 2024, researchers at the National Institutes of Health (NIH) applied artificial intelligence (AI) to a…
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…

On Apr. 7, 2024, Edwards Lifesciences announced the release of data from the HUDDLE study that examined the…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Apr. 5, 2024, groundbreaking was held for the Roy Blunt Soil Testing and Research Laboratory at the…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Apr. 2, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Apr. 1, 2024, Otsuka Pharmaceutica and Click Therapeutics announced that the U.S. Food and Drug Administration (FDA)…

On Apr. 1, 2024, the U.S. Department of Agriculture (USDA) confirmed the detection of highly pathogenic avian influenza…

On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported the first U.S. human…

On Mar. 29, 2024, the California Institute for Regenerative Medicine (CIRM) announced awards that support 11 projects in…

On Mar. 28, 2024, the American Chemical Society awarded The Priestley Medal to Carolyn R. Bertozzi “to recognize…

On Mar. 28, 2024, the Idaho State Department of Agriculture (ISDA) announced it had identified a highly pathogenic…

On Mar. 27, 2024, the Pecan Breeding and Genetics Program of the U.S. Department of Agriculture’s Agricultural Research…
On Mar. 27, 2024, the World Health Organization announced it had launched a network for coronaviruses, CoViNet, to…
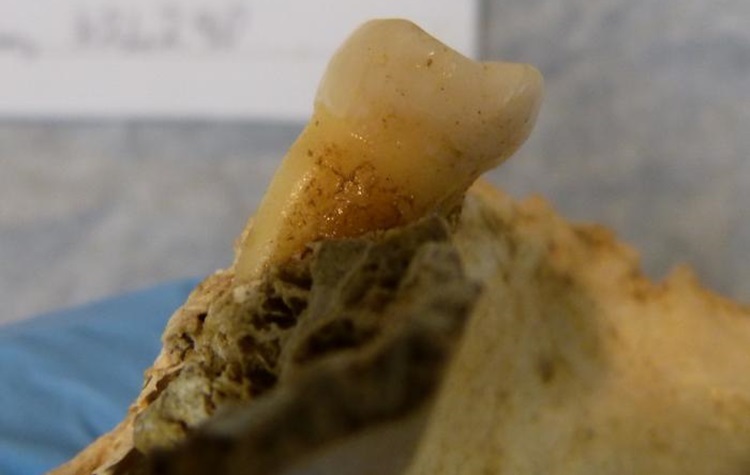
On Mar. 27, 2024, researchers from Trinity College Dublin announced they had recovered remarkably preserved microbiomes from two…

On Mar. 26, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had approved the cobas…
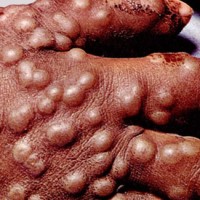
On Mar. 26, 2024, the National Academies of Sciences, Engineering, and Medicine released a report that said action…

On Mar. 25, 2024, immediately following the first detection of H5N1 in dairy cattle in the Texas panhandle…

On Mar. 22, 2024, the White House announced the creation of the National Bioeconomy Board. The Board will…

On Mar. 22, 2024, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for Pemgarda…

On Mar. 21, 2024, Yield10 Bioscience announced that USDA-APHIS’s Biotechnology Regulatory Services (“BRS”) has determined that Yield10’s Camelina…

On Mar. 21, 2024, Eledon Pharmaceuticals announced that tegoprubart, the company’s investigational anti-CD40L antibody, was used as a…

On Mar. 21, 2024, the U.S. Food and Drug Administration (FDA) approved Italfarmaco’s Duvyzat (givinostat) oral medication for…
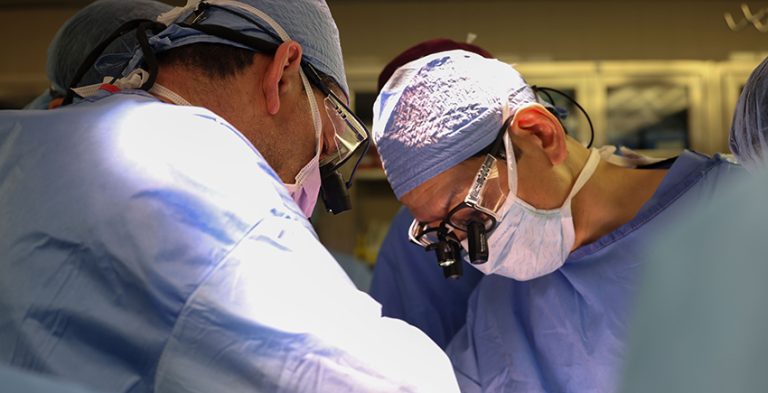
On Mar. 21, 2024, Harvard Medical School physician-scientists at Massachusetts General Hospital announced they had successfully transplanted a…

On Mar. 20, 2024, a National Institutes of Health-supported study reported that SARS-CoV-2, the virus that causes COVID-19,…

On Mar. 20, 2024, the U.S. Department of Agriculture (USDA) announced they had reviewed a University of Wisconsin…