Sanofi and GSK confirmed agreement with European Union to supply up to 300 million doses of adjuvanted COVID-19 vaccine
On Sept. 18, 2020, Sanofi and GSK finalised and signed an advanced purchase agreement with the European Commission…

On Sept. 18, 2020, Sanofi and GSK finalised and signed an advanced purchase agreement with the European Commission…

On Sept. 18, 2020, Uconn researchers, in a paper published in Nature Communications, validated the clinical feasibility of…

On Sept. 17, 2020, two Cornell University research teams, studying crop viruses and insecticides’ physiological effects on insects,…

On Sept. 17, 2020, Eli Lilly and Amgen announced a global antibody manufacturing collaboration to significantly increase the…

On Sept. 17, 2020, Rigel Pharma announced the start of a multicenter, Phase 2 trial to evaluate the…
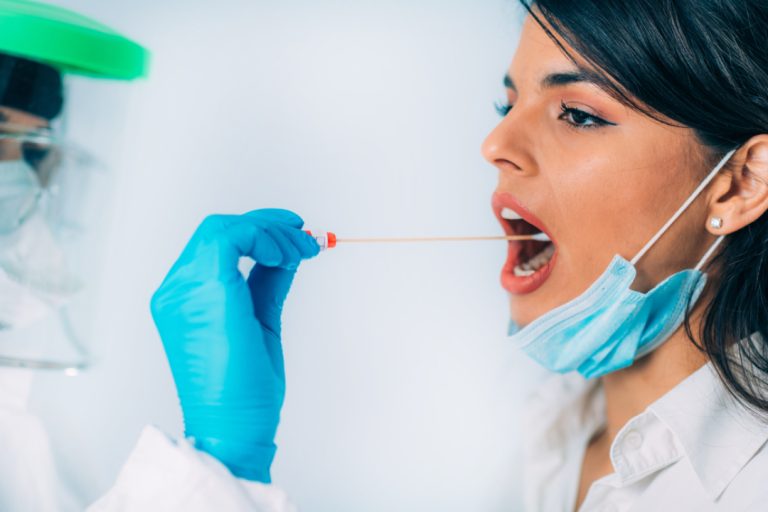
On Sept. 17, 2020, researchers announced a CRISPR-based test developed at MIT and the Broad Institute that can…

On Sept. 17, 2020, Quest Diagnostics announced that individuals can purchase a test to diagnose COVID-19 online, without…

On Sept. 17, 2020, Todos Medical announced that NLC Pharma had added the dietary supplement NLC-001 to its…

On Sept. 17, 2020, BioNTech announced an agreement with Novartis to acquire their GMP certified manufacturing facility in…

On Sept. 17, 2020, Atossa Therapeutics announced a positive interim safety assessment from the second cohort of healthy…

On Sept. 17, 2020, Roche announced that the phase III EMPACTA study met its primary endpoint, showing that…

On Sept. 16, 2020, the NIH announced a $12 million award for outreach and engagement efforts in ethnic…

On Sept. 16, 2020, Eli Lilly announced proof of concept data from an interim analysis of the BLAZE-1…
On Sept. 16, 2020, a study by researchers at La Jolla Institute for Immunology published in the Cell,…

On Sept. 16, 2020, NanoViricides announced that it had nominated a clinical drug candidate for the treatment of…

On Sept. 16, 2020, AIM ImmunoTech announced that recruitment had begun in Roswell Park Comprehensive Cancer Center’s Phase…
On Sept. 16, 2020, AXIM Biotechnologies announced that it had filed an Emergency Use Authorization (EUA) application with…

On Sept. 15, 2020, Novavax announced an amendment to its existing agreement with Serum Institute of India which…
On Sept. 15, 2020, initial findings reported by the Arizona COVID-19 Genomics Union (ACGU) suggested that following Arizona’s…
On Sept. 15, 2020, Innovation Pharma reported additional data from a U.S. Regional Biocontainment Laboratory (RBL) collected during…

On Sept. 15, 2020, the Tufts Center for the Study of Drug Development reported that new anti-infective drugs…

On Sept. 15, 2020, a team of scientists in the U.S. Department of Agriculture’s (USDA) Agriculture Research Service…

On Sept. 15, 2020, the FDA published comparative performance data for some authorized COVID-19 molecular diagnostic tests. The…
On Sept. 15, 2020, Vaxess Technologies and Medigen Vaccine Biologics (MBV) announced a partnership to develop a combined…

On Sept. 15, 2020, Thermo Fisher Scientific announced it was investing more than $140 million to further expand…
On Sept. 15, 2020, Soligenix announced publication of nonclinical results characterizing filovirus protein antigens (including for Ebola and…

On Sept. 15, 2020,Tonix Pharmaceuticals announced that the first patient was enrolled in the observational COV-LOGIC study (TNX-C001),…

On Sept. 15, 2020, OPKO Health announce it had initiated a Phase 2 trial with RAYALDEEᆴ as a…
On Sept. 15, 2020, BioNTech announced that had receive a grant of up to 375 million Euro from…

On Sept. 15, 2020, UT Southwestern research suggested conditions related to obesity, including inflammation and leaky gut, leave…