RedHill completed enrollment for COVID-19 US phase 2 study with opaganib
On Nov. 16, 2020, RedHill Biopharma announced that the U.S. Phase 2 study with opaganib (Yeliva, ABC294640)1 in…

On Nov. 16, 2020, RedHill Biopharma announced that the U.S. Phase 2 study with opaganib (Yeliva, ABC294640)1 in…

On Nov. 16, 2020, the UK Department for Health and Social Care Testing Innovation Fund announced £12.2M funding…

On Nov. 16, 2020, Biological E. Limited (BE), a Hyderabad-based vaccines and pharmaceutical company, Dynavax Technologies, and Baylor…
On Nov. 16, 2020, Moderna announced new data showing that mRNA-1273, its COVID-19 vaccine candidate, remained stable at…

On Nov. 16, 2020, Innovation Pharmaceuticals announced that an overseas Clinical Trial Application (CTA) has been submitted to…

On Nov. 16, 2020, Tonix Pharmaceuticals announced preliminary results following vaccination of non-human primates with TNX-1800 (modified horsepox…

On Nov. 16, 2020, BioNTech and Shanghai Fosun Pharmaceutica announced that the China National Medical Products Administration had…

On Nov. 16, 2020, INOVIO announced that it had received clearance from the the U.S. Food & Drug…

On Nov. 16, 2020, Aegis Sciences announced that it had launched a combined test for SARS-CoV-2 and influenza…

On Nov. 16, 2020, Moderna announced that the European Medicines Agency (EMA) human medicines committee (CHMP) had started…

On Nov. 15, 2020, Johnson & Johnson announced that it continued to enroll and vaccinate study participants for…

On Nov. 15, 2020, an independent data and safety monitoring board (DSMB) overseeing the Phase 3 trial of…
On Nov. 14, 2020, JAMA reported that in a preliminary study, adult outpatients with symptomatic COVID-19 treated with…

On Nov. 13, 2020, Roche announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Nov. 13, 2020, Merck announced that the U.S. Food and Drug Administration (FDA) had approved KEYTRUDA, Merck’s…

On Nov. 13, 2020, the NIIH announced that using a life support machine to replicate the functions of…
On Nov. 13, 2020, NeuroRx and Relief Therapeutics announced that to-date, 150 patients (out of a targeted enrollment…

On Nov. 13, 2020, Agilent Technologies announced it had received U.S. Food and Drug Administration (FDA) approval for…

On Nov. 13, 2020, Moderna announced that Swissmedic had started a rolling review of mRNA-1273, the Company’s vaccine…

On Nov. 13, 2020, a study from Washington University School of Medicine in St. Louis and St. Jude…

On Nov. 13, 2020, Albert Einstein College of Medicine announced that it had received a five-year, $4.9 million…

On Nov. 12, 2020, AstraZeneca announced that the CALAVI Phase II trials for Calquence (acalabrutinib) in patients hospitalised…

On Nov. 12, 2020, CureVac announced that its mRNA-based COVID-19 vaccine candidate, CVnCoV, remained stable and within defined…

On Nov. 12, 2020, the Bill & Melinda Gates Foundation announced new commitments totaling $70 million to global…

On Nov. 12, 2020 Vaxart announced additional results from its Hamster Challenge Study. The study evaluated Vaxartメs recombinant…

On Nov.12, 2020, the governing Board of the California Institute for Regenerative Medicine (CIRM) approved four new clinical…
On Nov. 12, 2020, Wren Laboratories announced that it had been granted an emergency use authorization by the…
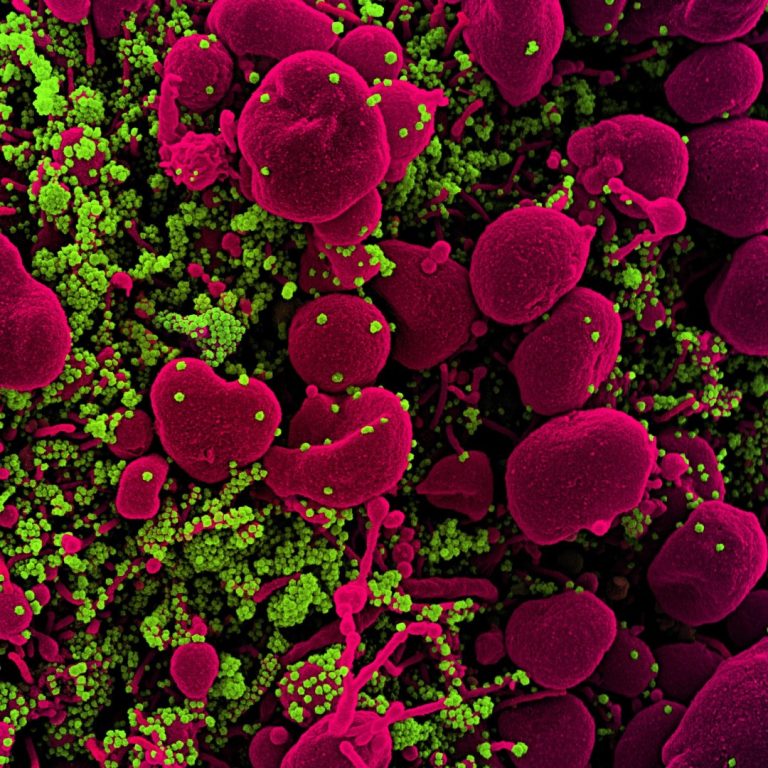
On Nov. 12, 2020, a study published in Science by a team of researchers in the U.S. and…

On Nov. 11, 2020, Moderna announced that it had completed case accrual for the first interim analysis of…

On Nov. 11, 2020, An international team of researchers with an effort called the Zoonomia Project announced that…