Study of ‘exceptional responders’ yields clues to cancer and potential treatments
On Nov. 19, 2020, in a comprehensive analysis of patients with cancer who had exceptional responses to therapy,…
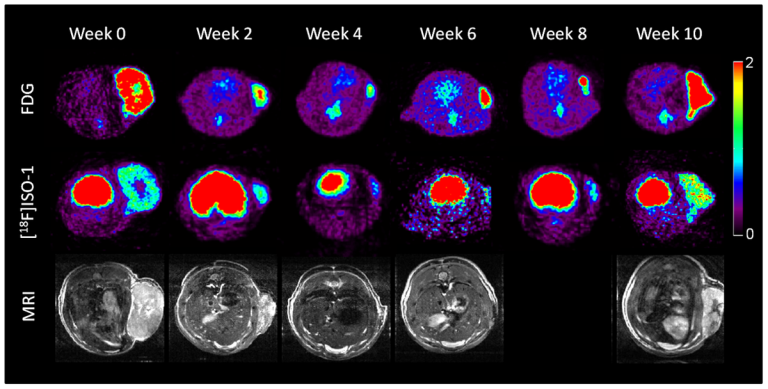
On Nov. 19, 2020, in a comprehensive analysis of patients with cancer who had exceptional responses to therapy,…

On Nov. 19, 2020, F4 Pharma announced the inclusion of the first patient with severe COVID-19 in a…

On Nov. 19, 2020, the University of Oxford announced that the ChAdOx1 nCov-2019 coronavirus vaccine had been shown…

On Nov. 19, 2020, University of Oxford researchers announced that research into the HIV-1 virus had shed light…

On Nov. 19, 2020, Novartis announced that it had entered into an exclusive worldwide license and collaboration agreement…
On Nov. 19, 2020, Medigen Vaccine Biologics announced that preclinical results of their COVID-19 vaccine candidate had been…

On Nov. 19, 2020, Cue Health announced that, as of November 9, the U.S. Department of Health and…

On Nov. 19, 2020, ApiJect Systems, a public benefit corporatio, announced that it had been approved by the…

On Nov. 19, 2020, Eli Lilly and Incyte announced that the FDA had issued an Emergency Use Authorization…

On Nov. 19, 2020, XBiotech announced data for its breakthrough candidate therapy for treating infections of influenza and…

On Nov. 19, 2020, Dallas-based company, Worlds Inc., the U.S. Air Force and Texas A&M University announced a…
On Nov. 18, 2020, marked the end of the 11th Ebola outbreak in the Democratic Republic of the…

On Nov. 18, 2020, BioVersys announced a milestone by entering clinical development with the start of Phase 1…

On Nov. 18, 2020, RELIEF THERAPEUTICS announced the appointment of Syneos Healthᆴ, a leading global clinical research organization…

On Nov. 18, 2020, Gilead Sciences announced topline results from the Phase 2/3 CAPELLA trial evaluating the company’s…

On Nov. 18, 2020, Pfizer and BioNTech announced that, after conducting the final efficacy analysis in their ongoing…

On Nov. 18, 2020, Todos Medical announced positive clinical proof of concept data from its lab-based rapid SARS-CoV-2…

On Nov. 18, 2020, Oregon Health & Science University (OHSU) announced an initiative that will attempt to discern…

On Nov. 17, 2020, the NIH announced funding of new research examining racial and ethnic disparities in pregnancy-related…

On Nov. 17, 2020, the U.S. Dept. of Veterans Affairs (VA) announced it was working with the Centers…

On Nov. 17, 2020, CureVac announced that it had accelerated the expansion of its manufacturing network to deliver…

On Nov. 17, 2020, the FDA issued an emergency use authorization (EUA) for the first COVID-19 diagnostic test…

On Nov. 17, 2020, RedHill Biopharma announced that the U.S. Food and Drug Administration (FDA) had cleared the…

On Nov. 17, 2020, the proposed settlements between the federal government and OxyContin maker Purdue Pharma and its…

On Nov. 17, 2020, Moderna announced a supply agreement with the government of the United Kingdom (UK) to…

On Nov. 17, 2020, the Mayo Clinic reported that more than 900 employees had contracted COVID-19 in the…

On Nov. 17, 2020, Celdara Medical announced that the National Institute of Allergy and Infectious Disease (NIAID) of…

On Nov. 17, 2020, UT Southwestern researchers reported that being younger doesnメt protect against the dangers of COVID-19…

On Nov. 16, 2020, BioNTech and Shanghai Fosun Pharmaceutica announced that the China National Medical Products Administration had…

On Nov. 16, 2020, INOVIO announced that it had received clearance from the the U.S. Food & Drug…