Scientists released a new human ‘pangenome’ reference
On May 10, 2023, researchers released a new high-quality collection of reference human genome sequences that captures substantially…

On May 10, 2023, researchers released a new high-quality collection of reference human genome sequences that captures substantially…
On May 9, 2023, United Nations agencies and partners reported that an estimated 13.4 million babies were born…

On May 9, 2023, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…

On May. 9, 2023, the U.S. Department of Agriculture announced that scientists have collaborated to produce the first…
On May 8, 2023, scientists at the National Institutes of Health (NIH) announced that they had identified new…
On May 5, 2023, France reported an increase in cases of severe neonatal sepsis associated with Enterovirus (Echovirus-11…
On May 5, 2023, the World Health Organization (WHO) ended its declaration of COVID-19 as a global health…
On May 5, 2023, the National Institutes of Health reported that a study had identified features of Long…

On May 3, 2023, Washington State University (WSU) made history in the United States (US) by becoming the…

On May 3, 2023, Vertex announced the Food and Drug Administration (FDA) ha approved KALYDECOᆴ (ivacaftor) for use…
On May 3, 2023, the U.S. FDA approved GlaxoSmithKline’s Arexvy as the first respiratory syncytial virus (RSV) vaccine…
On May 3, 2023, National Institutes of Health (NIH) scientists announced that they had uncovered new details on…

On May 2, 2023, the U.S. FDA announced that it was taking additional steps to support the use…
On May 2, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On May 2, 2023, Elanco Animal Health announced that the U.S. Department of Agriculture had provided a conditional…
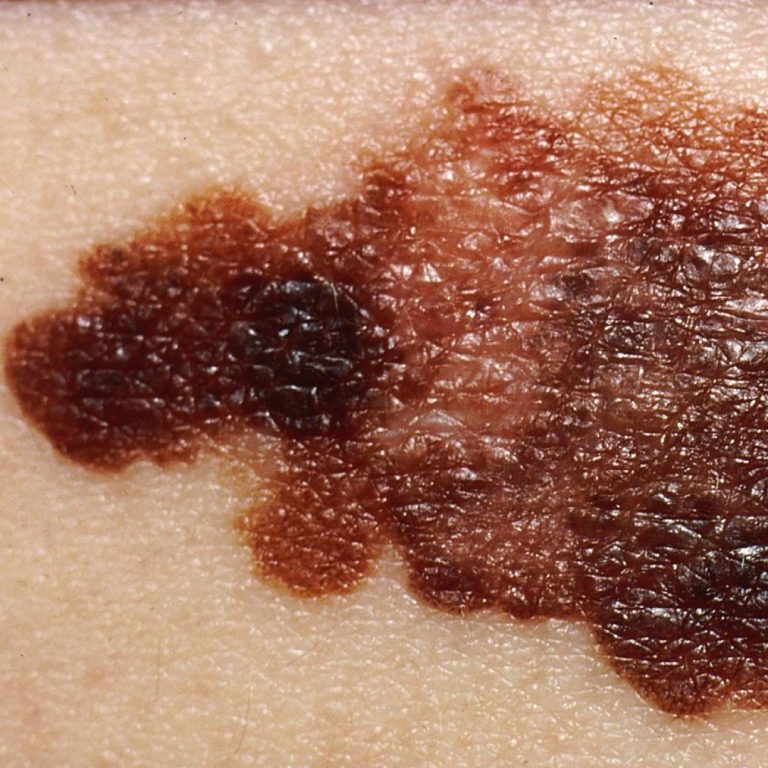
On May 1, 2023, an Oregon Health Sciences University (OHSU) dermatologist and a multi-disciplinary team confirmed that a…
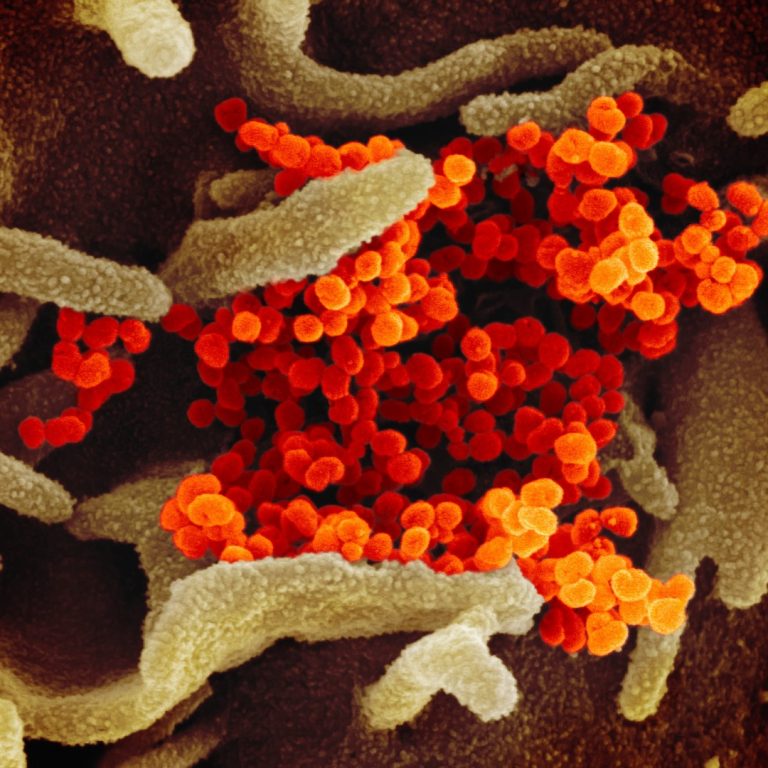
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…

On Apr. 27, 2023, Pfizer announced that experimental respiratory syncytial virus (RSV) vaccine was 82% effective in preventing…

On Apr. 27, 2023, the Zoonomia Project team announced in in a special issue of Science, that had…

On Apr. 27, 2023, researchers at Cornell University announced they had used ancient DNA extraction and analysis to…

On Apr. 26, 2023, Vertex announced the Food and Drug Administration (FDA) had approved the expanded use of…
On Apr. 25, 2023, the U.S. FDA approved Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis (ALS)…

On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…

On Apr. 24, 2023, Boehringer Ingelheim inaugurated its state-of-the-art Biologicals Development Center in Biberach an der Ri�, Germany….

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 20, 2023, Massachusetts Institute of Technology (MIT) researchers announced that have developed machine-learning algorithms that can…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Apr. 17, 2023, the U.S. FDA approved Gamida Cell’s Omisirge (omidubicel-onlv), a substantially modified allogeneic (donor) cord…

On Apr. 17, 2023, a new initiative led by Jennifer Doudna and Jill Banfield at the Innovative Genomics…

On Apr. 15, 2023, the World Health Organization (WHO) announced that six additional laboratory-confirmed cases of Marburg virus…