FDA conditionally approved first oral tablet to treat lymphoma in dogs
On Jan. 11, 2021, the FDA conditionally approved Anivive Lifesciences’ Laverdia-CA1 (verdinexor tablets) to treat dogs with lymphoma,…

On Jan. 11, 2021, the FDA conditionally approved Anivive Lifesciences’ Laverdia-CA1 (verdinexor tablets) to treat dogs with lymphoma,…

On Jan. 11, 2021, Editas Medicine announced the U.S. Food and Drug Administration (FDA) had cleared the initiation…

On Jan. 11, 2021, Baxter announced an agreement to provide sterile manufacturing services for NVX-CoV2373, Novavax COVID-19 recombinant…
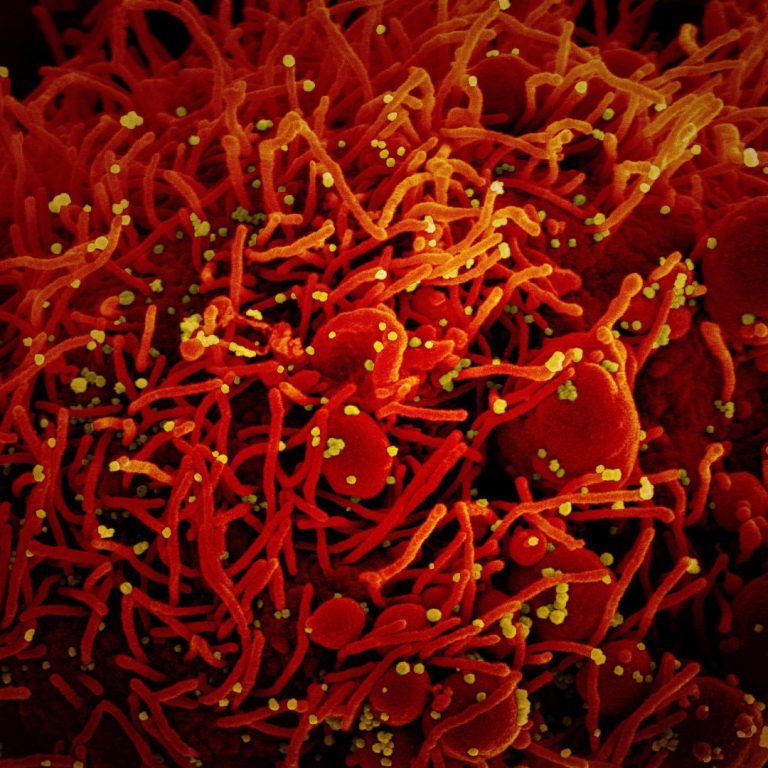
On Jan. 11, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel – a molecular diagnostic test that detects…

On Jan. 11, 2021, Moderna announced that it was expanding its pipeline of innovative vaccines with three new…
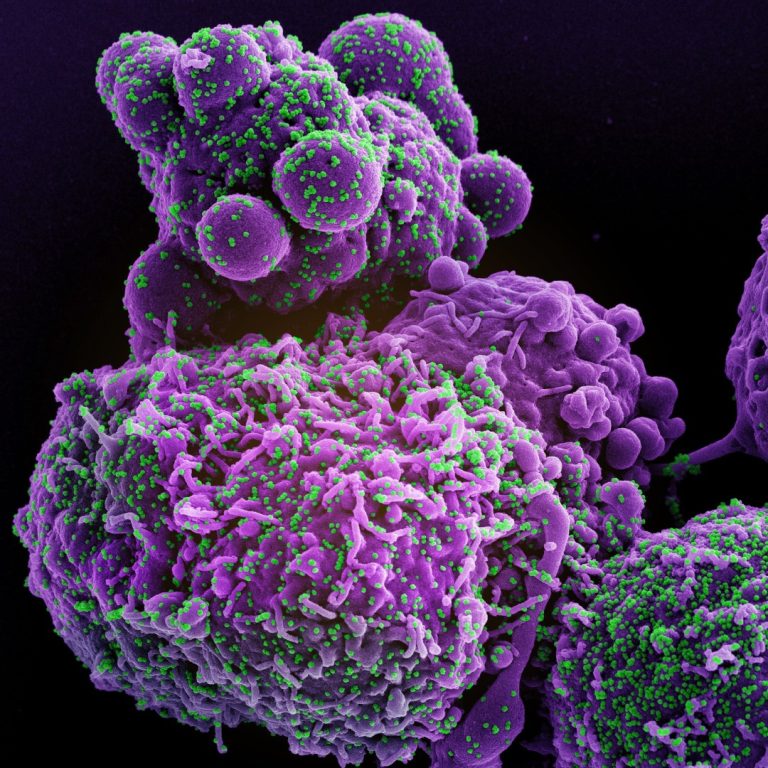
On Jan. 11, 2021, AXIM Biotechnologies announced that it had released a preprint of its manuscript describing the…

On Jan. 10, 2021, Humanigen announced that they were partnering to make lenzilumab available to hospitalized and hypoxic…

On Jan. 8, 2021, the American Cancer Society reported that the death rate from cancer in the U.S….

On Jan. 8, 2021, the FDA alerted clinical laboratory staff and health care providers that it was monitoring…

On Jan. 8, 2021, the COVID-19 vaccine developed by Moderna has today been given regulatory approval for supply…

On Jan. 8, 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) announced it had accepted the recommendation…

On Jan. 8, 2021, Pfizer and BioNTech announced results from an in vitro study conducted by Pfizer and…

On Jan. 8, 2021, BioNTech announced that it was in talks with the European Commission (EC) about an…

On Jan. 7, 2021, PerkinElmer and Oxford Immunotec announce an agreement under which PerkinElmer acquired Oxford Immunotec for…

On Jan. 7, 2021, Twist Bioscience announced it would supply the U.S. Centers for Disease Control and Prevention…

On Jan. 7, 2021, Oragenics announced entering into a material transfer agreement with Adjuvance Technologies for use of…

On Jan. 7, 2021, Novavax announced that it had executed an Advance Purchase Agreement with the Commonwealth of…

On Jan. 7, 2021, LabCorp announced that it had been awarded a contract from the Centers for Disease…

On Jan. 7, 2021, BioNTech announced publication of preclinical data on its novel mRNA vaccine approach against autoimmune…

On Jan. 6, 2021, the U.S. Department of Health and Human Services (HHS) announced the Centers for Disease…

On Jan. 6, 2021, the La Jolla Institute for Immunology announced data that suggest nearly all COVID-19 survivors…

On Jan. 6, 2021, Moderna announced that the European Commission had granted a conditional marketing authorization for COVID-19…

On Jan. 5, 2021, the National Institute of Allergy and Infectious Diseases (NIAID) announed that a phase 2/3…

On Jan. 5, 2021, Illumina and Helix announced a collaboration to augment national surveillance infrastructure in the U.S….

On Jan. 5, 2021, AIM ImmunoTech announced that the active AMP-511 Expanded Access Program (EAP) had dosed its…

On Jan. 4, 2021, Audrey Winkelsas, a 2015 NIH Oxford-Cambridge Scholars Program doctoral student with spinal muscle atrophy…

On Jan. 4, 2021, a research partnership between scientists at Scripps Research and UC San Diego found the…

On Jan. 4, 2021, Moderna announced a supply update for the Moderna COVID-19 Vaccine, increasing its base-case global…

On Jan. 4, 2021, Moderna announced that Israel’s Ministry of Health (MOH) had given authorization to import the…

On Jan. 4, 2021, Inovio Pharmaceuticals and Advaccine Biopharmaceuticals announced that they had entered into a collaboration and…