scientist-run Pathoplexus virus database vows to be transparently run and simple to use
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…
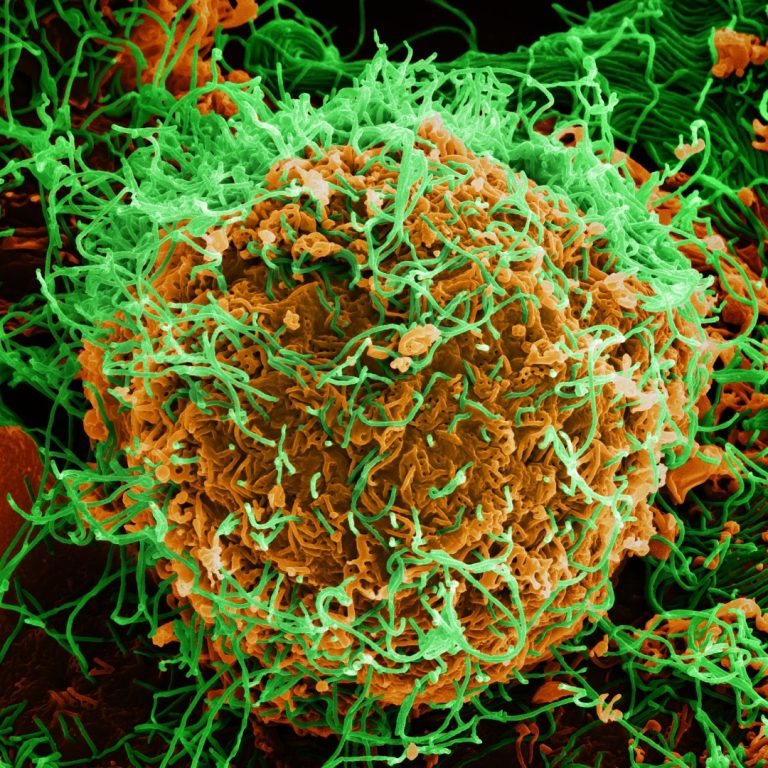
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…
![European Commission expanded Merck’s ERVEBO [Ebola Zaire vaccine] indication to include children 1 year of age and older](https://lifesciencehistory.com/wp-content/uploads/2022/08/european_commission_logo-768x768.jpg)
On Sept. 7, 2023, Merck announced that the European Commission (EC) had approved an expanded indication for ERVEBO…

On Aug. 3, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved an expanded…
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…

On Dec. 9, 2022, the World Health Organization (WHO) announced that the first doses of one of the…

On Nov. 11, 2021, the University of Oxford began recruiting for a Phase I trial to test an…
On Oct. 14, 2020, the FDA approved Regeneron Pharmaceutical’s Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn), a mixture of three…
On Oct. 14, 2020, Regeneron announced that the FDA had approved Inmazeb (atoltivimab, maftivimab and odesivimab-ebgn) for the…
On May 29, 2020, Johnson & Johnson announced that its Janssen Pharmaceutical subsidiary received a positive opinion from…

On May 26, 2020, Merck and and IAVI, a nonprofit scientific research organization dedicated to addressing urgent, unmet…
On Mar. 23, 2020, Soligenix announced that its ongoing collaboration with the University of Hawaii at Manoa (UH…
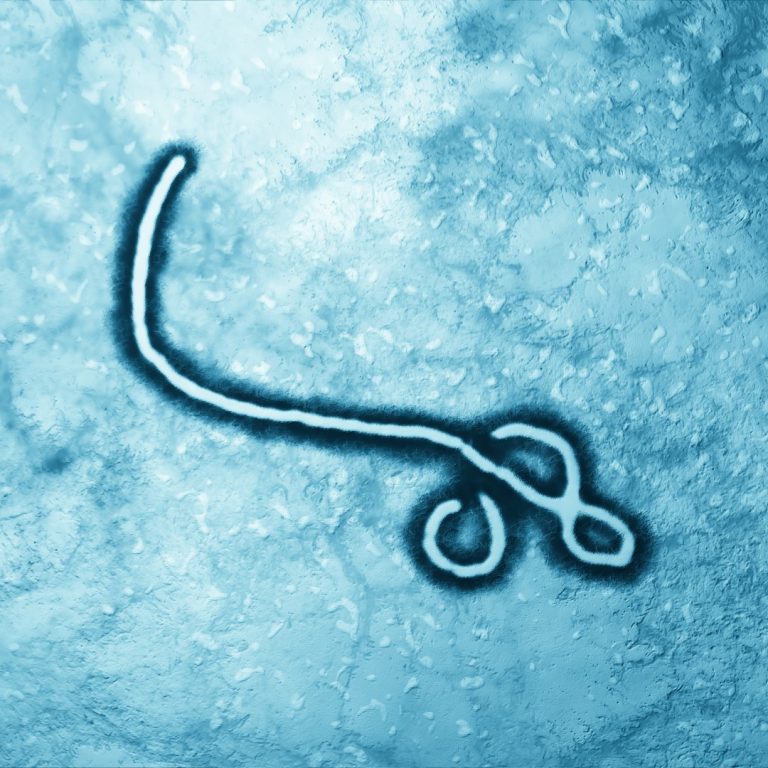
On May 6, 1995, the U.S. Centers for Disease Control and Prevention (CDC) investigated an deadly outbreak of…
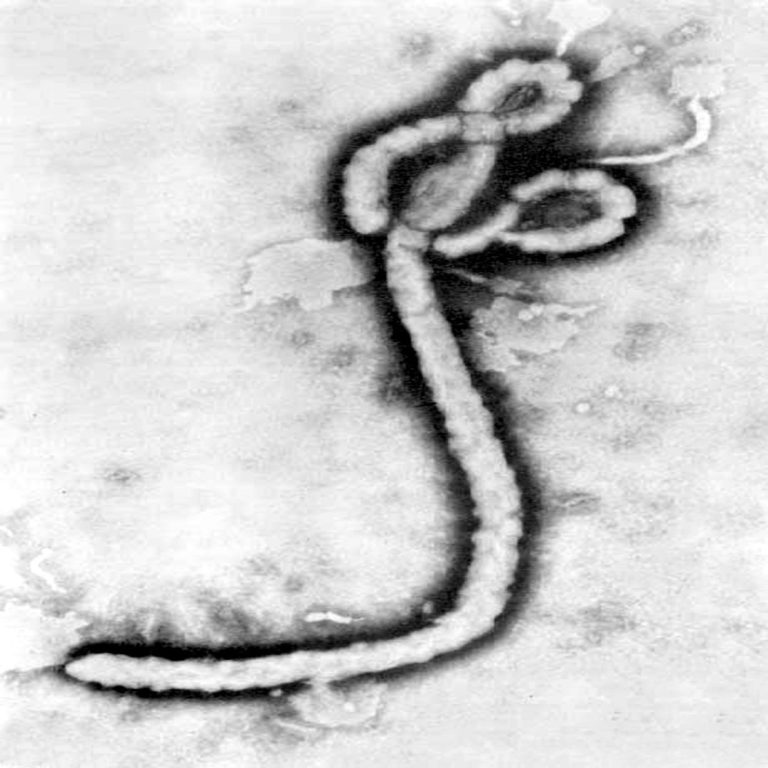
On Oct. 13, 1976, the Ebola virus was first identified in Sudan and Zaire (now Democratic Republic of…

In 1976, patients began presenting at a rural hospital in northwest Democratic Republic of Congo (then referred to…