NIH study to determine incidence of novel Coronavirus infection in US children began
On May 4, 2020, a NIH study to help determine the rate of novel coronavirus infection in children…

On May 4, 2020, a NIH study to help determine the rate of novel coronavirus infection in children…

On May 4, 2020, Cerus announced FDA regulatory approval for manufacture of INTERCEPT plasma with a new, alternative…

On May 4, 2020, NanoViricides announced that it has signed a Confidential Disclosure Agreement with a leading pharmaceutical…

On May 4, 2020, Junshi Biosciences and Eli Lilly announced an agreement to co-develop therapeutic antibodies for the…

On May 4, 2020, Arcturus Therapeutics and Catalent announced a partnership to support the expected manufacture of Arcturusメ…

On May 2, 2020, Roche announced that the FDA has issued an Emergency Use Authorization for its new…

On May 1, 2020, the U.S. Dept. of Veterans Affairs (VA) announced participation in a series of clinical…

On May 1, 2020, the CDC launched the SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology and Surveillance…

On May 1, 2020, Moderna and Lonza announced a 10-year strategic collaboration agreement to enable larger scale manufacture…

On Apr. 30, 2020, Eiger BioPharmaceuticals announced that the first patients have been dosed in a Phase 2…
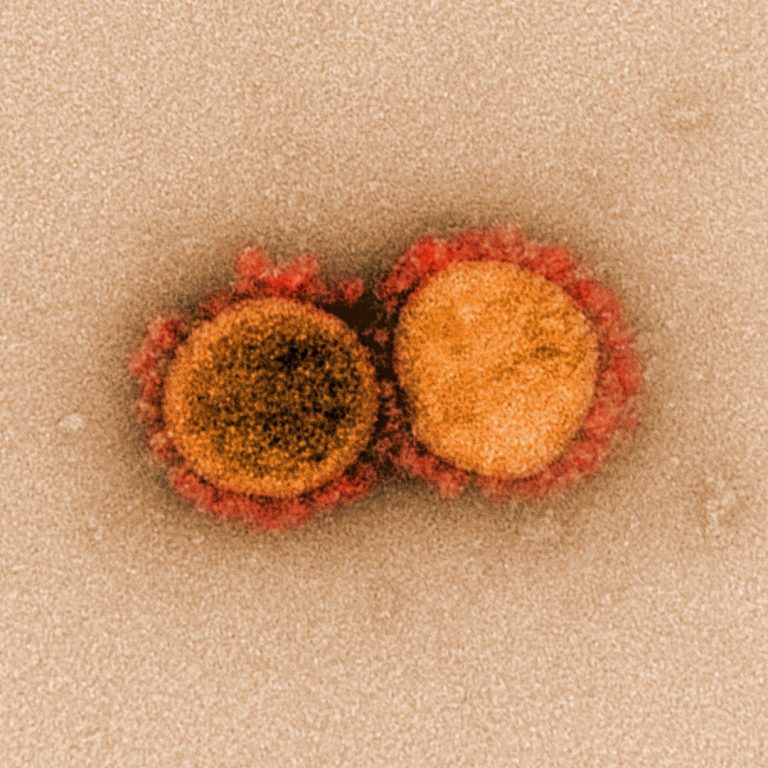
On Apr. 30, 2020, an international team of more than 120 scientists has detailed the impact of 75…

On Apr. 30, 2020, Emory University researchers are taking part in a multi-site study across the U.S. to…

On Apr. 30, 2020, AstraZeneca and the University of Oxford announced an agreement for the global development and…

On Apr. 30, 2020, the research community reacted with alarm and anger to the National Institutes of Health’s…

On Apr. 29, 2020, the U.S. Dep. of Veterans Affairs (VA) announced a partnership with the XPRIZE Foundationメs…

On Apr. 29, 2020, an anti-inflammatory drug developed at Scripps Research 25 years ago tested as a way…

On Apr. 29, 2020, Hologic announced that it planned to launch a new Aptimaᆴ molecular assay to detect…

On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine and NYU Langone…

On Apr. 28, 2020, Entos Pharmaceuticals announced a collaboration with EpiVax. This partnership combined the Entos Fusogenix DNA…

On Apr. 28, 2020, the Dutch consortium of AFPRO Filters, Royal Auping, and Royal DSM began large-scale production…

On Apr. 28, 2020, INOVIO and GeneOne Life Science announced interim data through week 16 from a Phase…

On Apr. 27, 2020, LabCorpᆴ announced that it will offer antibody tests for the virus that causes COVID-19….

On Apr. 27, 2020, a novel means to prevent HIV infection was developed at the University of Nebraska…

On Apr. 27, 2020, Diffusion Pharmaceuticals announced the pre-IND submission to the FDA of a planned clinical program…

On Apr. 27, 2020, Mateon Therapeutics announced announced it has submitted an Investigational New Drug application to the…

On Apr. 27, 2020, ARTES Biotechnology, the German-based biotech company specializing in process development for recombinant vaccines, entered…

On Apr. 27, 2020, Moderna announced it had submitted an Investigational New Drug application to the U.S. Food…

On Apr. 25, 2020, the World Health Organization (WHO) there was currently ‘no evidence’ that people who have…

On Apr. 24, 2020, a new study led by researchers at Oxford and Guangdong Centre for Diseases Control…

On Apr. 24, 2020, the governing Board of the California Institute for Regenerative Medicine (CIRM) awarded $749,999 to…