US, Chile and Peru interim trial data showed Oxford-AstraZeneca vaccine was safe and highly effective
On Mar. 22, 2021, Oxford University announced that a Phase III study of the Oxford-AstraZeneca coronavirus vaccine conducted…

On Mar. 22, 2021, Oxford University announced that a Phase III study of the Oxford-AstraZeneca coronavirus vaccine conducted…

On Mar. 21, 2021, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On Mar. 19, 2021, the FDA issued an emergency use authorization to Tiger Tech Solutions for the first…

On Mar. 17, 2021, Tonix Pharma announced preliminary results following vaccination of non-human primates with TNX-1800 (modified horsepox…

On Mar. 17, 2021, Altasciences working with the National Research Council of Canada (NRC), announced that it had…

On Mar. 15, 2021, ImmunityBio announced it had met the safety requirements for the first 12 participants in…

On Mar. 11, 2021, ImmunityBio announced it was developing a novel hAd5 ACE2 Decoy therapeutic vaccine to neutralize…

On Mar. 10, 2021, CEPI, the Coalition for Epidemic Preparedness Innovations, and VBI Vaccines announced a partnership to…

On Mar. 10, 2021, Innovation Pharmaceuticals announced that a Machine Learning (Artificial Intelligence) model used to screen 1,482…
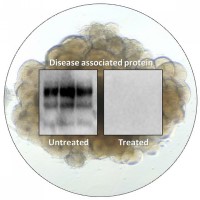
On Mar. 9, 2021, the NIH announced that approximately two years after establishing a human cerebral organoid system…

On Mar. 9, 2021, NanoViricides announced that NV-CoV-2 and NV-CoV-2-R were found to be highly effective against a…

On Mar. 9, 2021, VBI Vaccines announced the initiation of enrollment of its Phase 1/2 clinical study of…

On Mar. 8, 2021, ImmunityBio and NantKwest announced that the first cohorts of their South Africa and U.S….

On Mar. 5, 2021, Abbott announced the U.S. Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA) for…

On Mar. 5, 2021, Innovation Pharmaceuticals reported that eight sites were participating in the Company’s international Phase 2…

On Mar. 2, 2021, the National Institutes of Health (NIH), announced that it had launched a research effort…

On Mar. 2, 2021, Innovation Pharmaceuticals reported that eight sites were participating in the Companyメs international Phase 2…

On Mar. 2, 2021, the National Institutes of Health (NIH) halted a clinical trial evaluating the safety and…

On Mar. 1, 2021, a study from Washington University School of Medicine in St. Louis provided evidence that…

On Feb. 25, 2021, Altimmune announced that it had commenced enrollment in a Phase 1 clinical trial of…

On Feb. 24, 2021, Quest Diagnostics introduced a new COVID-19 testing service that aids in providing insight into…

On Feb. 23, 2021, the NIH announced that a randomized, placebo-controlled Phase 1 clinical trial of two monoclonal…

On Feb. 22, 2021, Biohaven Pharmaceutical announced that a hyperimmune globulin mimic (HGM) developed with Biohaven’s proprietary MATE…

On Feb. 20, 2021, Russian authorities reported the detection of influenza A(H5N8) virus infection in seven poultry workers…

On Feb. 18, 2021, Agilent Technologies announced the launch of the Agilent Dako SARS-CoV-2 IgG Enzyme-Linked Immunosorbent Assay…

On Feb. 18, 2021, Washington University pediatric infectious diseases doctors announced plans to launch clinical trials in the…

On Feb. 18, 2021, Pacific Biosciences announced that Labcorp had increased its commitment to highly accurate HiFi sequencing…
On Feb. 13, 2021, the University of Oxford, together with three partner sites in London, Southampton and Bristol,…
On Feb. 11, 2021, the WHO announced that COVID-19 cases and deaths surged in Africa as more contagious…

On Feb. 11, 2021, the Oregon Department of Agriculture (ODA) lifted the quarantine on the Oregon mink farm…