Riceland Foods was founded by a group of Arkansas rice farmers
In 1921, Riceland Foods was founded by a group of Arkansas rice farmers that created a farmers cooperative…

In 1921, Riceland Foods was founded by a group of Arkansas rice farmers that created a farmers cooperative…
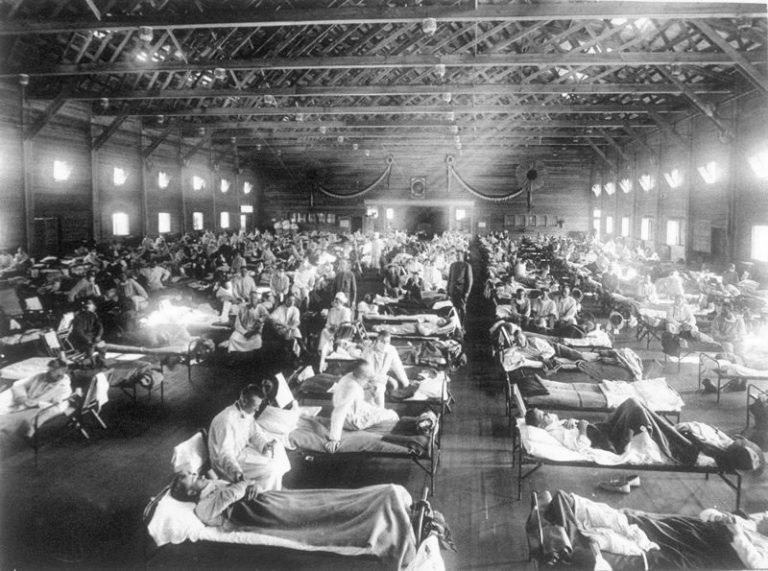
By the end of 1918, Dallas had lost a disputed number of lives to infleunza, between 250 and…

On Oct. 12, 1918, Dallas Mayor Lawther ordered all public and private schools, churches, and other public gatherings…

On Oct. 10, 1918, all Dallas theaters, playhouses, and all other places of public amusement were closed due…

By Oct. 9, 1918, over 1,000 cases of influenza had been reported in Dallas. Later that day, Mayor…

On Oct. 3, 1918, the Spanish Flu reached Portland, Oregon when Private James McNeese, a young soldier on…
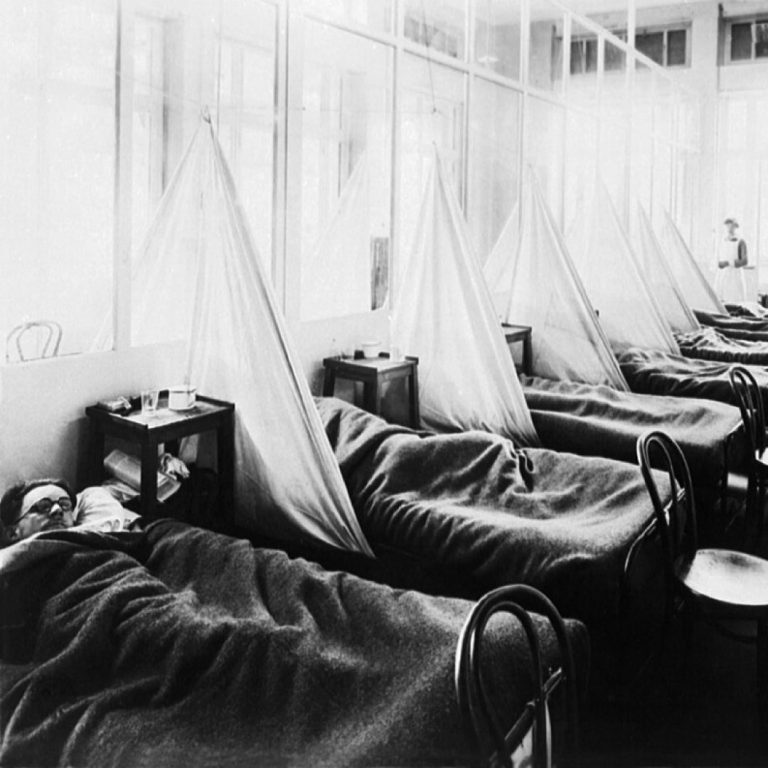
On Sept. 30, 1918, Texas health officials first reported that influenza was present in the state. By Oct….

By Sept. 27, 1918, influenza cases had arrived in the city of Dallas, prompting Dallas Health Officer Dr….

On Apr. 27, 1862, the Tariff Act, also known as the Dallas Tariff, exempted foreign plants and trees…