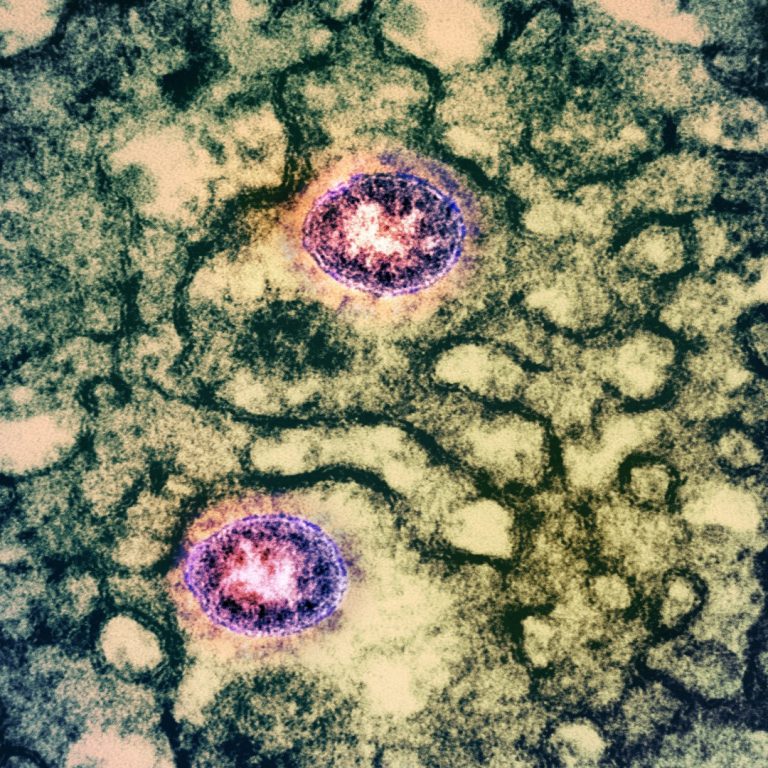
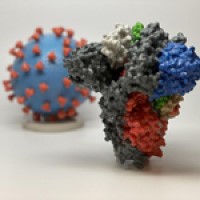
Icosavax announced topline interim results from its ongoing Phase 1/2 clinical trial of IVX-411, a VLP vaccine candidate displaying the SARS-CoV-2 receptor-binding domain (RBD).
On Mar. 25, 2022, Icosavax announced the first subjects had been dosed with IVX-411, a virus-like particle (VLP)…