Novavax and Serum Institute of India received Emergency Use Authorization for COVID-19 vaccine in the Philippines
On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…

On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Oct. 11, 2021, Inovio Pharmaceuticals announced that it had received authorization from Colombia’s INVIMA, to conduct the…

On Sept. 23, 2021, Novavax with its partner, Serum Institute of India announced a regulatory submission to the…

On Jul. 21, 2021, Humanigen announced its development and commercialization partners for South Korea and the Philippines, Telcon…

On Jun. 1, 2021, CytoDyn announced that Chiral Pharma in the Philippines placed its first purchase order for…

On May 21, 2021, Moderna announced that the Ministry of Food and Drug Safety of South Korea (MFDS)…

On Apr. 15, 2021, CytoDyn announced it had executed an exclusive supply and distribution agreement with Chiral Pharma…

On Mar. 29, 2021, CytoDyn announced that the Republic of the Philippines, Department of Health, Food and Drug…

On Mar. 22, 2021, Moderna announced that the Philippines had secured 7 million additional doses of COVID-19 Vaccine…

On Mar. 6, 2021, Moderna announced a supply agreement with the Government of the Philippines for 13 million…
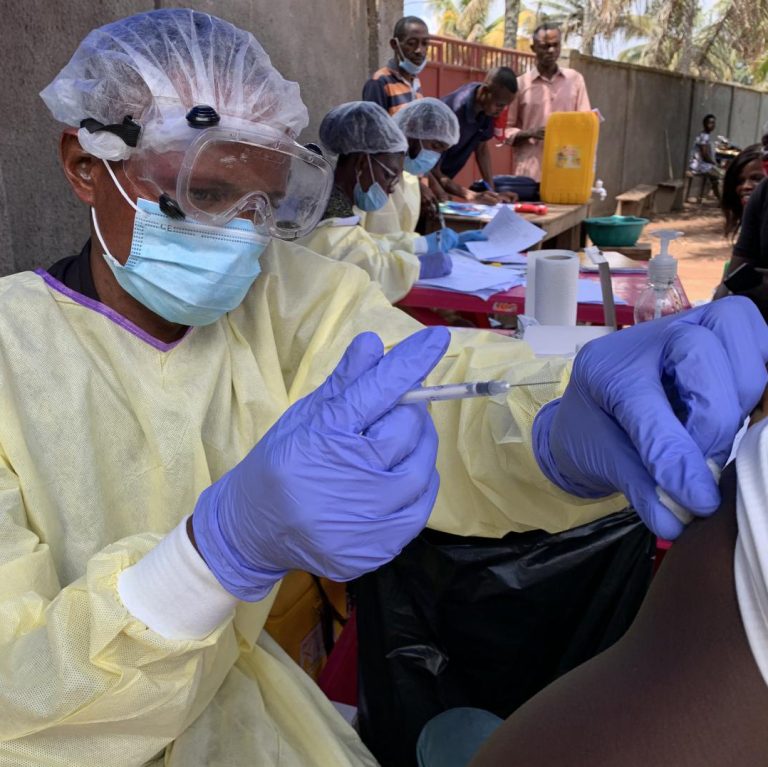
On Dec. 21, 2020, the U.S. National Institutes of Health (NIH) announced a study published in the Proceedings…

On Nov. 3, 2020, Humanigen announced the execution of its first licensing transaction in the Asia-Pacific Region with…

In 1982, having lost, neglected, or eaten to extinction most of its local rice varieties during the recent…

In 1966, a headline in the Manila Bulletin read MARCOS GETS MIRACLE RICE – the first time the…
In 1964, the International Rice Research Institute in the Philippines started the Green Revolution with new strains of…