‘Fire amoeba’ from Lassen Volcanic National Park survives in hotter conditions than any other complex cell
On Dec. 2, 2025, a team of microbiologist announced they discovered an organism at Lassen Volcanic National Park…

On Dec. 2, 2025, a team of microbiologist announced they discovered an organism at Lassen Volcanic National Park…

On Nov. 12, 2025, a study led by the Hebrew University of Jerusalem, and the Laboratory of Entomology…

On Jul. 17, 2025, the U.S. Centers for Disease Control and Prevention (CDC) released a report about an…

On May 23, 2025, the U.S. Centers for Disease Control and Prevention, University of Alaska Anchorage, ans Colorado…

On Mar. 28, 2025, Visby Medical™ announced that the U.S. Food and Drug Administration (FDA) has granted De…

On Jan. 15, 2025, the California Department of Public Health (CDPH) reported thatqOne confirmed human infection with influenza…

On Nov. 21, 2024, scientists at the University of Florida (UF) announced they have developed a pioneering tool…
On Jul. 9, 2024, Twist Bioscience announced the expansion of its growing portfolio of synthetic viral controls with…

On Jun. 10, 2024, Roche announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…
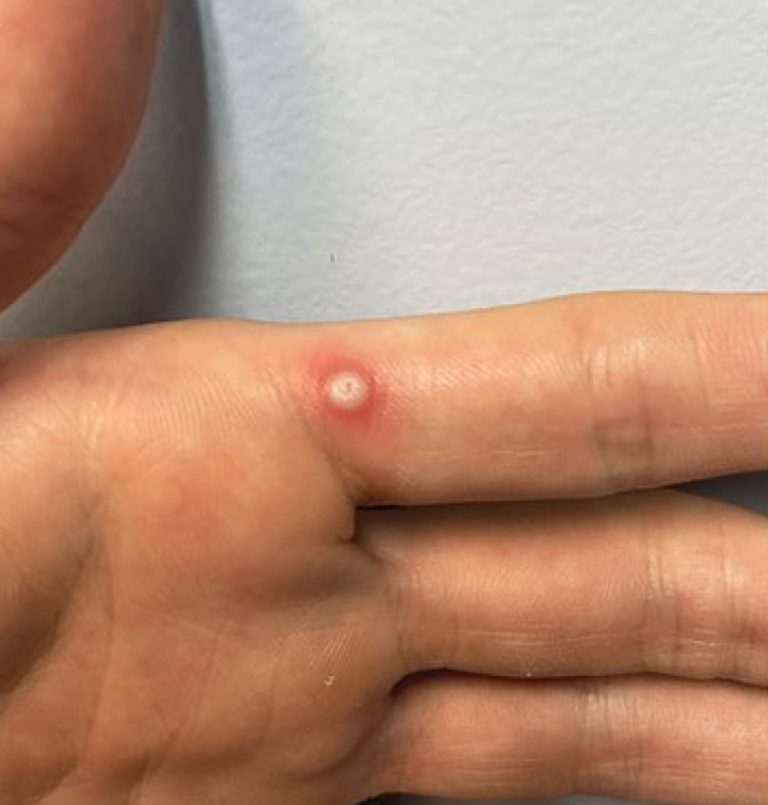
On Apr. 10, 2024, LabCorp announced that the U.S. Food and Drug Administration (FDA) had granted Emergency Use…
On Jul. 24, 2023, the United Arab Emirates (UAE), notified WHO of a case of Middle East Respiratory…
On Jan. 25, 2023, Roche and its subsidiary TIB Molbiol announced they had developed a COVID-19 PCR test…
On Jan. 9, 2023, BD (Becton, Dickinson) and CerTest Biotec have announced Emergency Use Authorization (EUA) from the…
On Nov. 15, 2022, Roche announced that the U.S. Food and Drug Administration had granted Emergency Use Authorization…

On Oct. 7, 2022, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to…
On Jul. 27, 2022, BD (Becton, Dickinson) announced their newly developed molecular polymerase chain reaction (PCR) test for…

On Jul 14. 2022, Aegis Sciences announced it is was authorized to begin testing for monkeypox using the…

On Jul 13. 2022, Quest Diagnostics announced the nationwide availability of the company’s lab-developed molecular diagnostic test to…

On Jun. 15, 2022, Roche announced that the US Food and Drug Administration had issued Emergency Use Authorization…

On May 26, 2022, BD (Becton, Dickinson) announced a new high-throughput molecular diagnostic combination test for SARS-CoV-2 and…
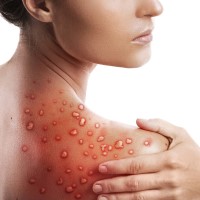
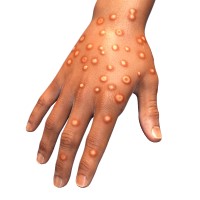
On May 21, 2022, the World Health Organization (WHO) reported that since May 13, 2022, cases of mpox…

On Mar. 30, 2022, Lucira Health announced that preliminary clinical trial results for its candidate COVID-19 & Flu…

On Feb. 24, 2022, Novavax shared extended analysis from its pivotal Phase 3 clinical trial conducted in the…
On Dec. 27, 2021, Sorrento Therapeutics announced that initial testing of COVISTIX on recombinant N proteins demonstrated its…

On Dec. 1, 2021, Fulgent Genetics confirmed that the Companyメs RT-PCR test for SARS-CoV-2, the virus that causes…
On Nov. 30, 2021, LabCorp announced that it had begun offering observed self-collection for COVID-19 PCR testing in…

On Nov. 29, 2021, Thermo Fisher Scientific confirmed that its polymerase chain reaction (PCR) TaqPath COVID-19 Combo Kit…
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Sept. 1, 2021, Quidel announced it will make Quidelメs non-prescription QuickVueᆴ At-Home OTC COVID-19 Test available to…

On Aug. 6, 2021, BD (Becton, Dickinson) and CerTest Biotec announced the CE mark for a molecular test…