Arbutus Biopharma announced research initiative to identify novel antiviral therapies for treatment of Coronavirus infections
On May 11, 2020, Arbutus Biopharma announced the establishment of a new research initiative focused on the identification…
On May 11, 2020, Arbutus Biopharma announced the establishment of a new research initiative focused on the identification…

On May 11, 2020, a study was published in JAMA Network that attempts to answer the question whether…

On May 11, 2020, in a perspective published by the journal Science, Dr. Larry Corey from the Fred…
On May 8, 2020, the Rice University COVID-19 Research Fund Oversight and Review Committee announced it had funded…

On May 8, 2020, Abbott announced new research, published in the Journal of Clinical Microbiology, which found that…

On May 8, 2020, the U.S. Patent and Trademark Office (USPTO) announced a new COVID-19 Prioritized Examination Pilot…

On May 8, 2020, the NIH announced that a randomized, controlled clinical trial evaluating the safety and efficacy…

On May 8, 2020, the U.S. Food and Drug Administration (FDA) issued the first emergency use authorization (EUA)…

On May 8, 2020, the FDA issued an emergency use authorization (EUA) to Rutgers Clinical Genomics Laboratory for…

On May 8, 2020, the Council of Scientific and Industrial Research (CSIR) under its flagship programme NMITLI announced…

On May 8, 2020, Quidel announced it had received Emergency Use Authorization (EUA) from the FDA to market…

On May 8, 2020, BioReference Laboratories, an OPKO Health company, announced the launch of COVID-19 antibody screening available…

On May 7, 2020, the Defense Health Agency announced that the Air Force Genetics Center of Excellence at…

On May 7, 2020, Washington University in St. Louis announced that people with COVID-19 who are seen by…

On May 7, 2020, the NIH announced a new online survey launched by the NIH-supported Rare Diseases Clinical…

On May 7, 2020, academics at the University of Oxford and the London School of Hygiene & Tropical…

On May 7, 2020, the Wellcome Sanger Institute announced that Roser Vento-Tormo, a Group Leader was one of…
On May 7, 2020, City of Hope announced that two existing drugs using advanced genomic technology, were being…
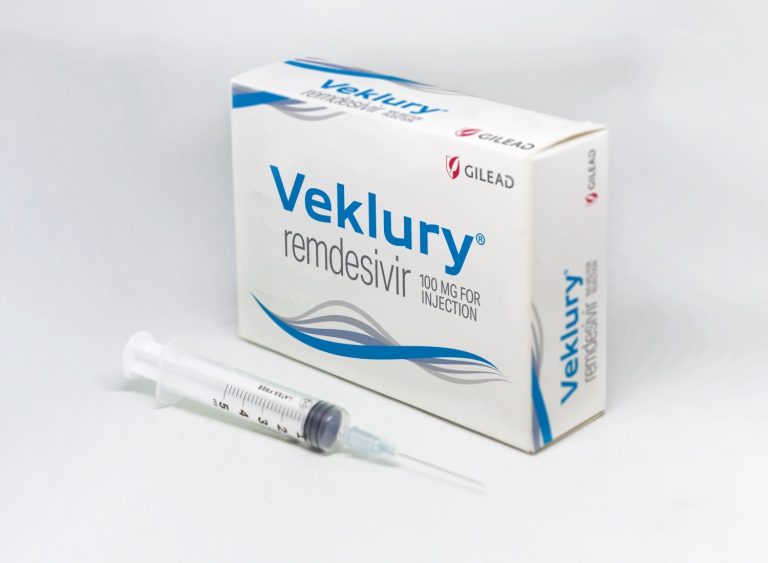
On May 7, 2020, Gilead announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) has granted…

On May 7, 2020, OPTI Medical Systems, an IDEXX Laboratories subsidiary announced the U.S. Food and Drug Administration…

On May 7, 2020, Rebekah Gundry, Ph.D., was one of only 12 investigators nationwide to receive a “COVID-19…

On May 7, 2020, Tonix Pharmaceuticals and the University of Alberta announced a research collaboration and exclusive licensing…

On May 7, 2020, a study of nearly 1,400 patients with moderate to severe COVID-19 disease at a…

On May 7, 2020, the CoVId-19 Plasma Alliance, an unprecedented plasma industry collaboration recently established to accelerate the…

On May 6, 2020, Humanigen announced that the first COVID-19 patient had been dosed in its previously announced…

On May 6, 2020, 3M and Ford announced that newly designed powered air-purifying respirators (PAPRs), developed are on…

On May 6, 2020, Predictive Oncology announced the acquisition of Soluble Therapeutics and partnership and licensing of a…

On May 6, 2020, Meridian Bioscience announced a collaboration with QuantuMDx on its newly launched SARS-CoV-2 assay for…

On May 6, 2020, Mesoblast announced that the first patients were dosed in the 300-patient randomized placebo-controlled Phase…

On May 6, 2020, Sinovac Biotech announced publication of the preclinical study on animals for its vaccine candidate…