WHO and Wikimedia Foundation expanded access to trusted information about COVID-19 on Wikipedia
On Oct. 22, 2020, the World Health Organization (WHO) and the Wikimedia Foundation announced a collaboration to expand…
On Oct. 22, 2020, the World Health Organization (WHO) and the Wikimedia Foundation announced a collaboration to expand…
On Oct. 22, 2020, IAVI, a nonprofit scientific research organization dedicated to addressing urgent, unmet global health challenges,…

On Oct. 22, 2020, Amyris and the Infectious Disease Research Institute (IDRI) announced the signing of a Collaboration…

On Oct. 22, 2020, Moderna announced that it had completed enrollment of 30,000 participants for the Phase 3…
On Oct. 21, 2020, ViiV Healthcare, majority owned by GSK, with Pfizer and Shionogi Limited as shareholders, announced…

On Oct. 21, 2020, ImmunityBio and NantKwest announced that the first patient had been dosed in the Phase…

On Oct. 21, 2020, CerTest Biotec, along with BD (Becton, Dickinson), announced that the VIASURE SARS-CoV-2 (N1 +…

On Oct. 20, 2020, the NIH announced that the researchers had completed the Adaptive COVID-19 Treatment Trial (ACTT-1),…

On Oct. 20, 2020, BioMedomics announced that the National Institute for Food and Drug Surveillance (INVIMA) had provided…
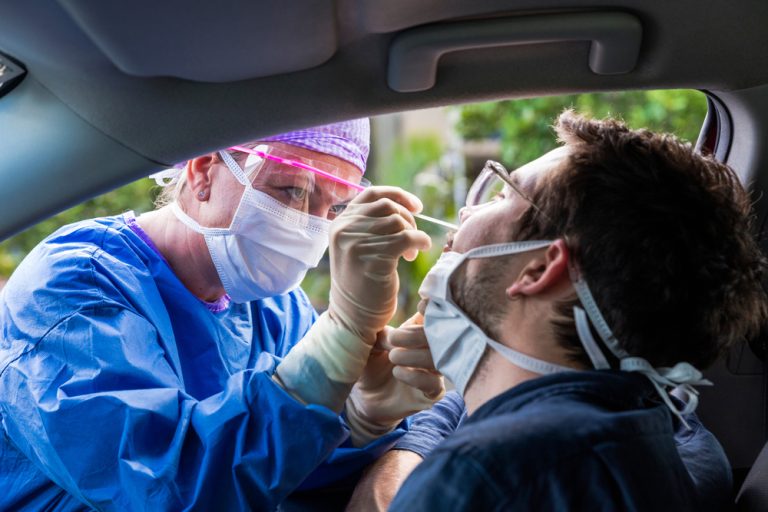
On Oct. 20, 2020, BioReference Laboratories, an OPKO Health company, announced that it was accepting specimens for a…

On Oct. 20, 2020, the European Investment Bank (EIB) and Immunic announced the signing of a タ24.5 million…

On Oct. 20, 2020, researchers at Oregon Health & Science University (OHSU) and Oregon State University (OSU) reported…

On Oct. 19, 2020, ICON announced that it had been re-selected by the Biomedical Advanced Research and Development…

On Oct. 19, 2020, Evotec announced that its Seattle-based subsidiary, Just – Evotec Biologics had received a grant…

On Oct. 19, 2020, Mateon Therapeutics announced the receipt of approval from Republica Argentina Poder Ejecutivo Nacional, the…

On Oct. 19, 2020, Thermo Fisher Scientific announced it had received an expansion of its Emergency Use Authorization…

On Oct. 19, 2020, LabCorp announced a test that provided a quantitative measurement of an individualメs SARS-CoV-2 IgG…

On Oct. 16, 2020, the NIH launched an adaptive Phase 3 clinical trial to evaluate the safety and…
On Oct. 16, 2020, BD (Becton, Dickinson) announced the CE mark and European availability of a product for…

On Oct. 16, 2020, the U.S. Department of Defense in coordination with the Department of Health and Human…

On Oct. 15, 2020, the World Health Organization (WHO) announced Interim that results from the Solidarity Therapeutics Trial,…
On Oct. 15, 2020, scientists from the University of Oxford’s Department of Physics announced they had developed an…

On Oct. 15, 2020, Eiger BioPharmaceuticals announced results of the ILIAD Study (Interferon Lambda for Immediate Antiviral Therapy…

On Oct. 15, 2020, ImmunityBio announced it had received authorization from the FDA to begin a Phase I…

On Oct. 15, 2020, Ionis Pharmaceuticals announced that IONIS-PKK-LRx was being evaluated in an investigator-initiated Phase 2 clinical…

On Oct. 15, 2020, Tonix Pharmaceuticals announced that the first participant was enrolled in the observational PRECISION study…

On Oct. 14, 2020, Moderna announced that it had received written confirmation from the European Medicines Agency (EMA)…

On Oct. 14, 2020, 3M and Discovery Education announced they had named 14-year-old Anika Chebrolu from Frisco, Texas,…

On Oct. 14, 2020, Tevogen Bio announced that its Investigational New Drug (IND) application to develop a COVID-19…

On Oct. 14, 2020, Pfizer and BioNTech announced that preliminary, peer-reviewed data from the Phase 1 portion of…