WHO recommended R21/Matrix-M vaccine for malaria prevention
On Oct. 2, 2023, the World Health Organization (WHO) announced it had recommended a new vaccine, R21/Matrix-M, developed…
On Oct. 2, 2023, the World Health Organization (WHO) announced it had recommended a new vaccine, R21/Matrix-M, developed…
On Sept. 28, 2023, the National Institute of health announced SARS-CoV-2, the virus that causes COVID-19, can directly…

On Sept. 28, 2023, Pfizer and BioNTech that Health Canada had authorized the companies’ Omicron XBB.1.5-adapted monovalent COVID-19…

On Sept. 27, 2023, Gritstone bio announced that it was awarded a contract by the Biomedical Advanced Research…
On Sept. 27, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that new diagnoses of…

On Sept. 11, 2023, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the supplemental Biologics…

On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Sept. 6, 2023, Moderna announced that clinical trial data from its research assay confirmed its updated COVID-19…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Aug. 29, 2023, the COVID-19 Technology Access Pool (C-TAP), a multi-stakeholder partnership to facilitate sharing of intellectual…

On Aug. 23, 2023, the CDC announced it had detected a new SARS-CoV-2 variant labeled BA.2.86. CDC was…
On Aug. 22, 2023, the U.S. Department of Health and Human Services (HHS) announced it had awarded more…
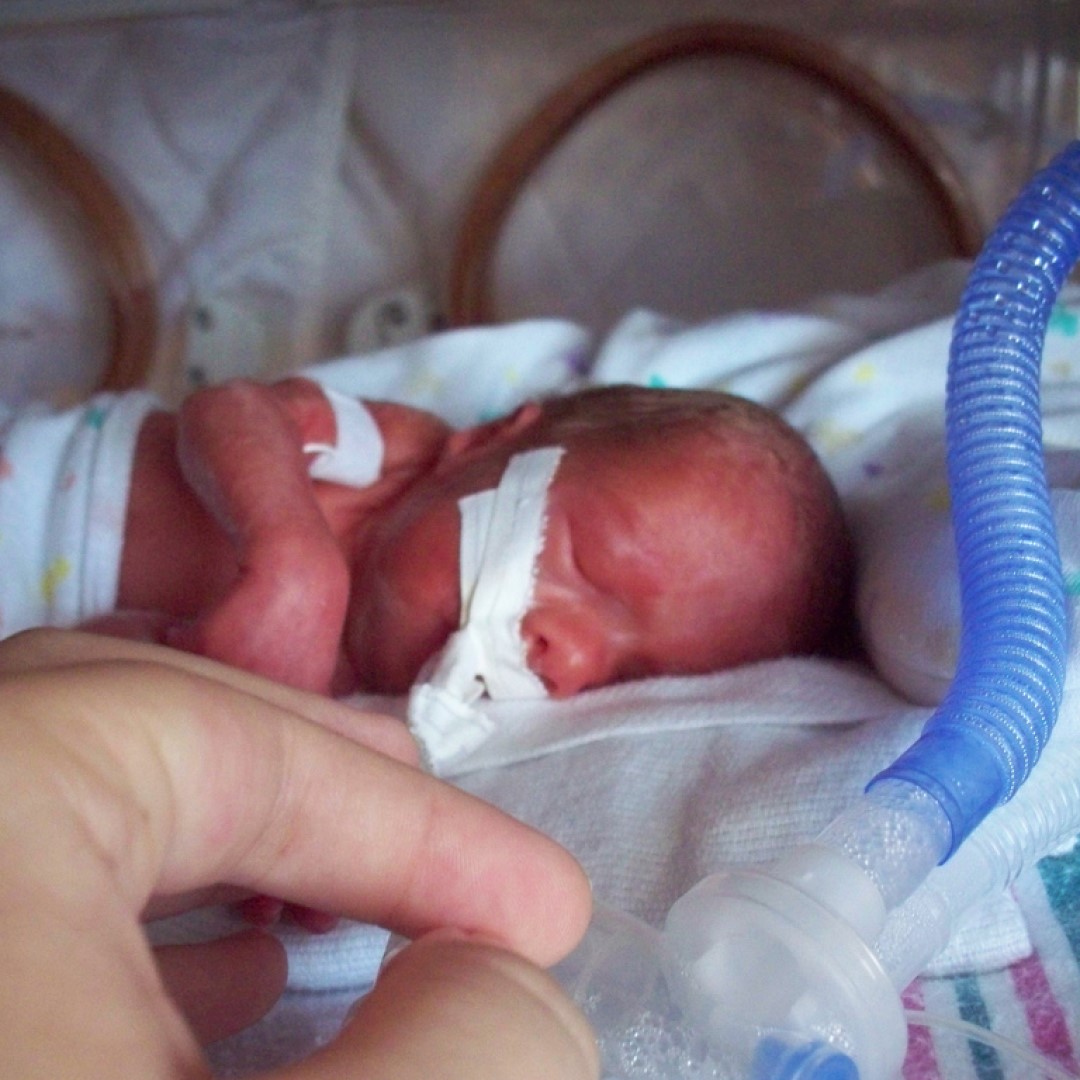
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…
On Aug. 9, 2023, the World Health Organization (WHO) released standing recommendations for battling COVID-19 and released an…
On Jul. 31, 2023, scientists at Washington University in St. Louis have developed a breath test that quickly…

On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…
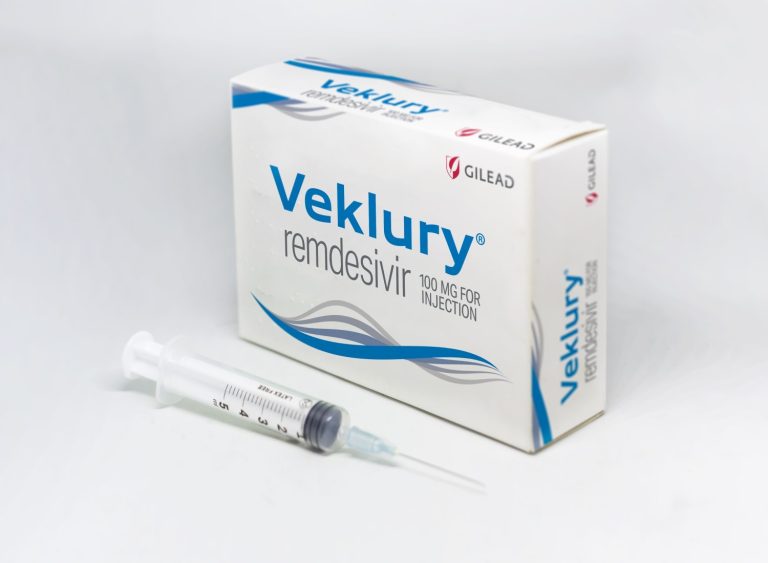
On Jul. 14, 2023, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Jul. 13, 2023, the Centers for Disease Control and Prevention (CDC) announced the launch of the Bridge…
On Jun. 16, 2023, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…
On Jun. 6, 2023, the U.S. Food and Drug Administration (FDA) granted granted marketing authorization for the Cue COVID-19…
On May 31, 2023, the World Health Organization announced it had assigned simple, easy to say and remember…

On May 26, 2023, Novavax announced that Nuvaxovid (NVX-CoV2373) had been recommended for full Marketing Authorization (MA) for…

On May 25, 2023, the U.S. Food and Drug Administration (FDA) approved the oral antiviral Paxlovid (nirmatrelvir tablets…

On May 25, 2023, the National Institutes of Health announced Initial findings from a study of nearly 10,000…

On May 19, 2023, HealthPartners Institute researchers have published new data in JAMA Network Open that shows monovalent…
On May 11, 2023, a National Institutes of Health supported long-term study reported that among people who have…

On May 11, 2023, the U.S. Departments of Health and Human Services announced an end of the COVID-19…
On May 5, 2023, the World Health Organization (WHO) ended its declaration of COVID-19 as a global health…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…
On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…