Johnson & Johnson announced agreement with Gavi to supply COVID-19 vaccine candidate to lower-income countries in 2021
On Dec. 18, 2020, Johnson & Johnson announced that its subsidiary Janssen Pharma, will provide up to 500…

On Dec. 18, 2020, Johnson & Johnson announced that its subsidiary Janssen Pharma, will provide up to 500…

On Dec. 18, 2020, Moderna announced that the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on…

On Dec. 17, 2020, NIAID announced that two randomized, controlled Phase 3 clinical trials had begun evaluating investigational…

On Dec. 17, 2020, XPhyto Therapeutics and 3a-diagnostics announced the successful validation of their point-of-care SARS-CoV-2 (COVID-19) RT-PCR…
On Dec. 17, 2020, JAMA reported that the daily U.S. mortality rate for COVID-19 deaths was equivalent to…
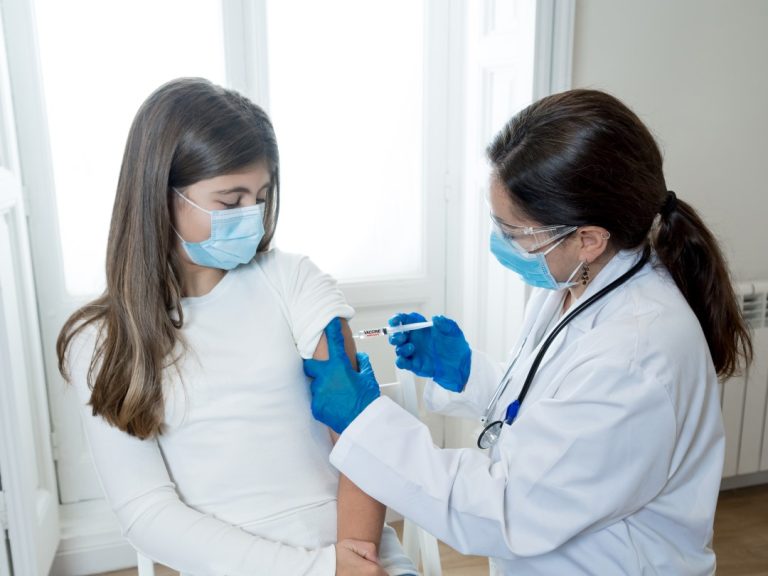
On Dec. 17, 2020, Moderna confirmed that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological…

On Dec. 17, 2020, Quest Diagnostics and The Commons Project, a non-profit public trust dedicated to building and…

On Dec. 17, 2020, PRA Health Sciences announced the enhancement of its COVID-19 Monitoring Program with the addition…

On Dec. 17, 2020, Johnson & Johnson announced that the large-scale, pivotal, multi-country Phase 3 trial (ENSEMBLE) of…

On Dec. 17, 2020, Regeneron announced that the New England Journal of Medicine (NEJM) had published initial clinical…

On Dec. 16, 2020, Abbott announced that the FDA had issued Emergency Use Authorization (EUA) for virtually guided…

On Dec. 16, 2020, the U.S. Food and Drug Administration issued a new emergency use authorization (EUA) for…

On Dec. 16, 2020, Novavax announced an Advance Purchase Agreement with the government of New Zealand for the…

On Dec. 16, 2020, Heat Biologics announced it had completed its gp96-based COVID-19 vaccine cell line, which is…

On Dec. 16, 2020, Pacific Biosciences announced initial findings from the companyメs research collaboration with Labcorp that was…

On Dec. 16, 2020, BD (Becton, Dickinson) announced that it had received pandemic orders for needles and syringes…

On Dec. 16, 2020, BioNTech and Shanghai Fosun Pharmaceutical announced an agreement to supply Mainland China with an…

On Dec. 15, 2020, Inovio Pharma announced the company and a team of scientists from The Wistar Institute,…

On Dec. 15, 2020, a months long study to determine the number of Houstonians carrying COVID-19 antibodies revealed…

On Dec. 15, 2020, Abbott announced it received had CE Mark for its new quantitative SARS-CoV-2 IgG (Immunoglobulin…
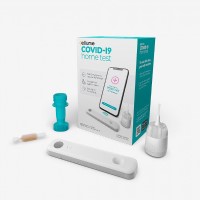
On Dec. 15, 2020, the FDA granted emergency use authorization (EUA) for the first over-the-counter (OTC) fully at-home…

On Dec. 15, 2020, TGen, an affiliate of City of Hope announced that it had identified a specific…

On Dec. 15, 2020, Aurinia Pharmaceuticals announced the funding and initiation of an open-label exploratory trial evaluating the…
On Dec. 15, 2020, AXIM Biotechnologies announced the development and patent filing for an enzyme-linked immunosorbent assay (ELISA)-based…

On Dec. 14, 2020, CureVac announced that it had enrolled the first participant in the pivotal Phase 2b/3…

On Dec. 14, 2020, Novartis announced that the Phase III RUXCOVID study evaluating ruxolitinib on top of standard…

On Dec.14, 2020, neuroscientists at University College London (UCL) announced they had found no significant association between COVID-19…
On Dec. 14, 2020, the U.S. Dept. of Veterans Affairs (VA) announced that the New Orleans and Bedford,…

On Dec. 14, 2020, Codagenix and the Serum Institute of India announced that a Phase 1 clinical trial…
On Dec. 14, 2020, Incyte announced that the Phase 3 RUXCOVID study evaluating the safety and efficacy of…