Moderna announced publication in The NEJM of interim results from phase 1 study of its mRNA vaccine against COVID-19
On Sept. 29, 2020, Moderna announced the publication of the second interim analysis of the open-label Phase 1…
On Sept. 29, 2020, Moderna announced the publication of the second interim analysis of the open-label Phase 1…
On Sept. 17, 2020, researchers announced a CRISPR-based test developed at MIT and the Broad Institute that can…

On Sept. 9, 2020, Harvard Medical School investigators at Massachusetts General Hospital reported that patients hospitalized with severe…
On Sept. 8, 2020, the CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Novavax, Pfizer, and…

On Sept. 2, 2020, Broad Institute of MIT and Harvard announced it had partnered with 108 public and…

On Aug. 28, 2020, Moderna confirmed that the Company was engaged in discussions with the Ministry of Health,…

On Aug. 25, 2020, data from the Broad Institute of MIT and Harvard, Massachusetts General Hospital, the Massachusetts…

On Aug. 24, 2020, humans are not the only species facing a potential threat from SARS-CoV-2, the novel…

On Aug. 20, 2020, in the most comprehensive study of COVID-19 pediatric patients to date, Harvard Medical School…

On Aug. 19, 2020, Johnson & Johnson announced an agreement to acquire Momenta Pharmaceuticals, a company that discovers…

On Aug. 4, 2020, Yale University announced it was among the core partners of the new Center for…

On Jul. 28, 2020, Moderna announced a preclinical study evaluating mRNA-1273, its vaccine candidate against COVID-19, was published…

On Jul. 27, 2020, the National Institutes of Health (NIH) announced that a phase 3 clinical trial designed…
On Jul. 27, 2020, Moderna announced that the Phase 3 study of its mRNA vaccine candidate (mRNA-1273) against…

On Jul. 26, 2020, Moderna announced a modification to its contract with the Biomedical Advanced Research and Development…

On Jul. 21, 2020, National Institutes of Health (NIH) researchers reported early results from the first COVID-19 vaccine…
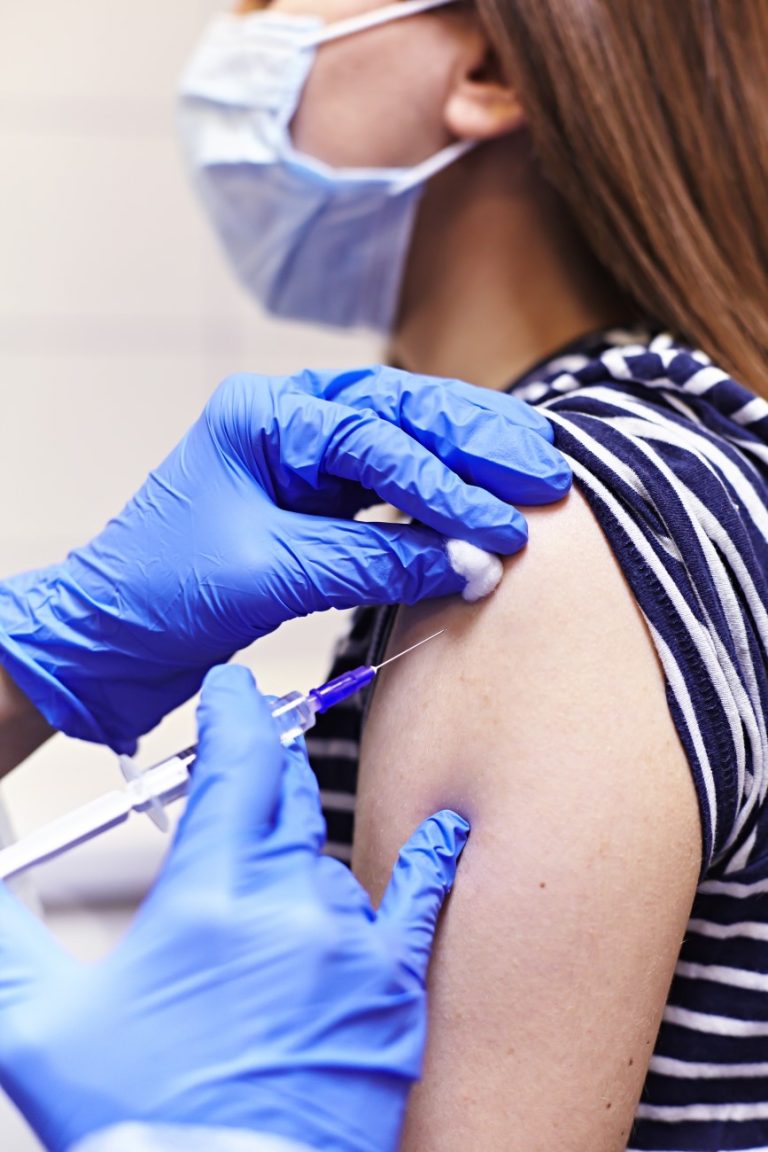
On Jul. 21, 2020, researchers from the National Institutes of Health (NIH) announced that early results from the…

On Jul. 14, 2020, Moderna announced the publication of an interim analysis of the open-label Phase 1 study…

On Jul. 9, 2020 Moderna and Laboratorios Farmaceuticos Rovi announced a collaboration for large-scale, commercial fill-finish manufacturing of…

On Jul. 8, 2020, Moderna announced that it had completed enrollment for both cohorts of the Phase 2…

On Jul. 8, 2020, Constant Therapeutics reported that its peptide drug TXA127 was tested in a series of…

On Jun. 25, 2020 Moderna announced a collaboration for large-scale, commercial fill-finish manufacturing of Moderna’s mRNA-based COVID-19 vaccine…

On Jun. 11, 2020, Moderna announced progress on late-stage development of mRNA-1273, the Company’s mRNA vaccine candidate against…

On May 29, 2020, Moderna announced the first participants in each age cohort had been dosed in the…

On May 28, 2020, Biogen and the Lemelson-MIT Program (LMIT) at the Massachusetts Institute of Technology announced the…

On May 18, 2020, Moderna announced positive interim clinical data of mRNA-1273, its vaccine candidate against novel coronavirus…

On May 13, 2020, the Massachusetts Consortium on Pathogen Readiness (MassCPR), a multi-institutional initiative convened by Harvard Medical…

On May 12, 2020, Moderna announced the U.S. Food and Drug Administration (FDA had granted Fast Track designation…

On May 12, 2020, the Broad Instituteメs Drug Repurposing Hub announced that it had opened its repository of…

On May 5, 2020, Massachusetts Eye and Ear and Massachusetts General Hospital (MGH), members of Mass General Brigham,…