Moderna finalized agreement with Government of the Republic of Kenya to establish an mRNA manufacturing facility
On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…

On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…
On Mar. 20, 2023, some of the world’s leading makers of flu vaccines said they could make hundreds…

On Feb. 24, 2023, Moderna announced will make certain contingent development, commercial and regulatory milestone payments to the…

On Feb. 15, 2023, Moderna announced it will continue to offer its COVID-19 vaccines for free, even after…

On Dec. 21, 2022, Moderna announced the the finalization of a strategic partnership with the United Kingdom (UK)…

On Dec. 8, 2022, Moderna announced it had received emergency use authorization from the U.S. Food and Drug…

On Oct. 31, 2022, Moderna announced that it had received approval from the Ministry of Health, Labour and…

On Oct. 19, 2022, the U.S. Food and Drug Administration (FDA) recommended the use of the Novavax COVID-19…

On Oct. 19, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 19, 2022, Moderna announced new clinical data on its bivalent Omicron-containing booster, mRNA-1273.214. Ninety days after…

On Oct. 17, 2022, Moderna and Gavi, the Vaccine Alliance, announced they had mutually agreed to cancel remaining…

On Oct. 12, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Aug. 23, 2022, Moderna announced that the Government of Canada had exercised its option to purchase an…

On Aug. 15, 2022, Moderna announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK…

On Aug. 9, 2022, Moderna announced an amendment to its agreement with the European Commission (EC) to convert…

On Jul. 29, 2022, the U.S. Department of Health and Human Services (HHS), in collaboration with the U.S….

On Jul. 22, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Jul. 18, 2022, Moderna announced that the Therapeutic Goods Administration (TGA) in Australia had granted provisional registration…

On Jul. 14, 2022, Moderna announced that Health Canada had approved the use of Moderna’s mRNA COVID-19 vaccine,…
On Jul. 11, 2022, the National Institute of Allergy and Infectious Diseases (NIAID) launched an early-stage clinical trial…

On Jun. 17, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Jun. 8, 2022, Moderna announced new clinical data on its Omicron-containing bivalent COVID booster candidate, mRNA-1273.214, containing…

On Jun. 2, 2022, Moderna announced an agreement with the European Commission (EC) to amend their originally agreed…

On May 31, 2022, Moderna and Takeda announced the transfer of the marketing authorization for Moderna’s COVID-19 vaccine…

On May 24, 2022, scientists at the Broad Institute of MIT and Harvard and the University of Massachusetts…

On May 19, 2022, the U.S. National Institutes of Health (NIH) announced that people who reported in a…

On May 18, 2022, Moderna and the nonprofit scientific research organization IAVI announced that the first participant screenings…
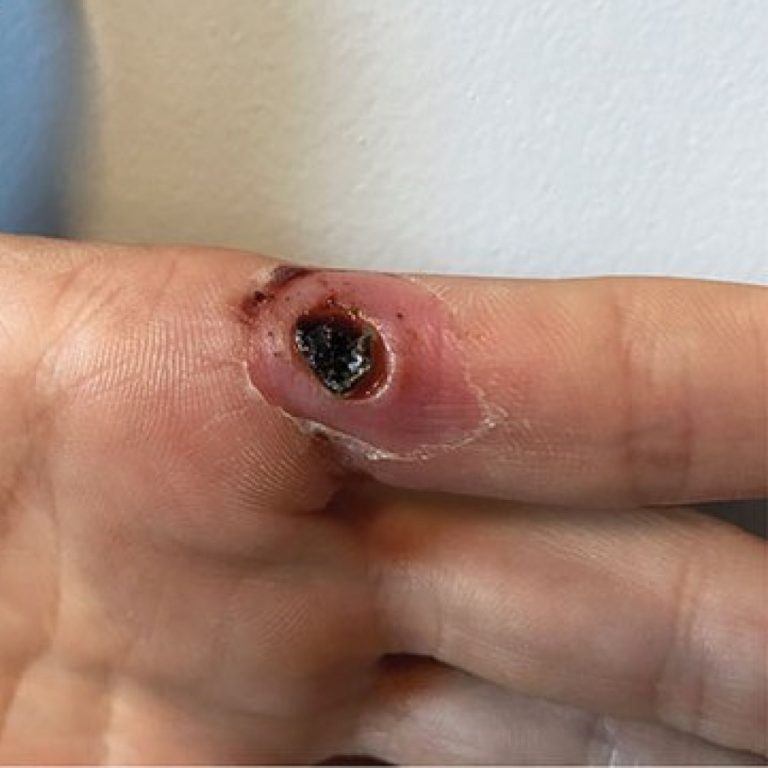
On May 18, 2022, scientists at the Centers for Disease Control and Prevention (CDC) announced that they were…

On Apr. 29, 2022, Moderna announced its plan to build a state-of-the-art mRNA vaccine manufacturing facility in Quebec…

On Apr. 29, 2022, Moderna announced that it had submitted for a variation to the conditional marketing authorization…