Novavax announced MOU to produce COVID-19 vaccine made in Canada
On Feb. 2, 2021, Novavax announced a memorandum of understanding (MOU) with the Canadian government to produce NVX-CoV2373,…

On Feb. 2, 2021, Novavax announced a memorandum of understanding (MOU) with the Canadian government to produce NVX-CoV2373,…

On Jan. 28, 2021, Novavax announced that NVX-CoV2373, its protein-based COVID-19 vaccine candidate, had met the primary endpoint,…

On Jan. 22, 2021, Novavax announced that it had finalized an agreement with the Government of Canada to…

On Jan. 11, 2021, Baxter announced an agreement to provide sterile manufacturing services for NVX-CoV2373, Novavax COVID-19 recombinant…

On Jan. 7, 2021, Novavax announced that it had executed an Advance Purchase Agreement with the Commonwealth of…

On Dec. 29, 2020, the U.S. Department of Defense (DOD) announced that the Womack Army Medical Center was…

On Dec. 29, 2020, University of Nebraska Medical Center (UNMC) and Nebraska Medicine announced participation in a clinical…

On Dec. 28, 2020, Novavax announced initiation of PREVENT-19, its pivotal Phase 3 study in the U.S. and…

On Dec. 16, 2020, Novavax announced an Advance Purchase Agreement with the government of New Zealand for the…

On Nov. 30, 2020, Novavax announced that two of the three planned late-stage efficacy trials for NVX-CoV2373 sponsored…
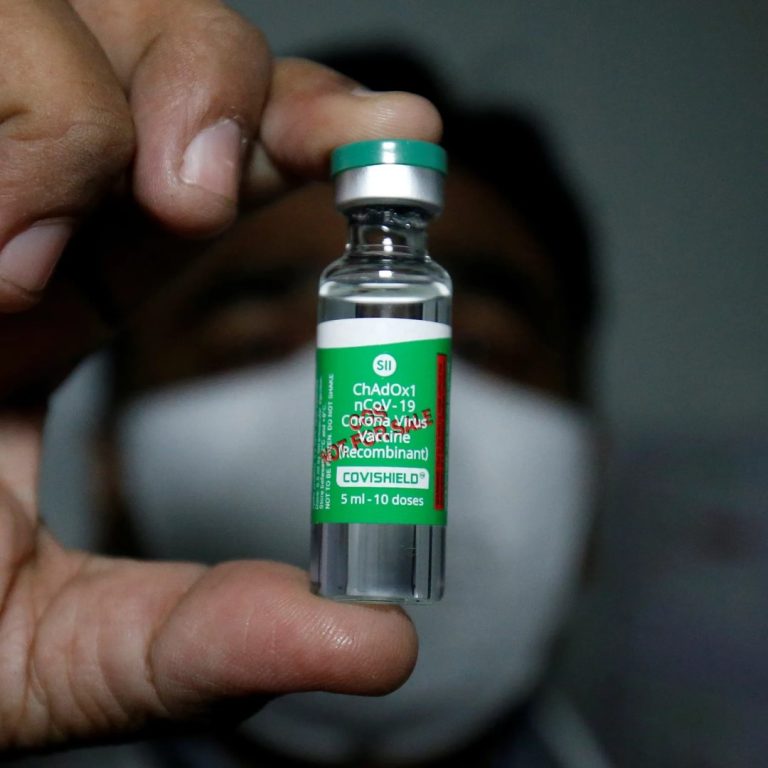
On Nov. 11, 2020, the Serum Institute of India (SII) and Indian Council of Medical Research (ICMR) announced…

On Nov. 4, 2020, Novavax announced the signing of a non-binding Heads of Terms document with the Australian…

On Nov. 2, 2020, Novavax announced the expansion of its Maryland campus to accommodate the company’s rapid growth…

On Oct. 27, 2020, Novavax announced updates on its Phase 3 clinical development program of NVX-CoV2373, its COVID-19…

On Sept. 24, 2020, Novavax announced that it had initiated its first Phase 3 study to evaluate the…

On Sept. 15, 2020, Novavax announced an amendment to its existing agreement with Serum Institute of India which…
On Sept. 8, 2020, the CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Novavax, Pfizer, and…

On Sept. 2, 2020, Novavax announced the publication in The New England Journal of Medicine of Phase 1…

On Aug. 31, 2020, Novavax announced it had reached an agreement in principle with the Government of Canada…

On Aug. 24, 2020, Novavax announced that the first volunteers were enrolled in the Phase 2 portion of…

On Aug. 24, 2020, Catalent announced that it had contracted to provide drug substance manufacturing to AstraZeneca for…

On Aug. 17, 2020, Novavax announced the beginning of a Phase 2b clinical trial in South Africa to…

On Aug. 13, 2020, Novavax and SK bioscience announced a development and supply agreement for the antigen component…

On Aug. 7, 2020, Novavax and Takeda Pharmaceutical announced a partnership for the development, manufacturing and commercialization of…

On Aug. 6, 2020, Novavax announced it had entered a supply and license agreement with the Serum Institute…

On Aug. 4, 2020, Novavax announced Phase 1 data from its Phase 1/2 randomized, observer-blinded, placebo-controlled trial of…

On Aug. 1, 2020, Novavax announced it had received approval to start human trials in India for the…

On Jul. 23, 2020, Novavax and FUJIFILM Diosynth Biotechnologies announced an agreement to manufacture bulk drug substance for…

On Jul. 22, 2020, Altimmune announced an agreement with Vigene Biosciences to manufacture AdCOVIDTM, Altimmuneメs single-dose intranasal vaccine…

On Jul. 9, 2020, a study from the NIH showed that neurofilament light chain (NfL) delivered superior diagnostic…