RSV vaccine highly effective against hospital admission in older adults, study suggests
On Nov. 12, 2025, UK researchers reported a test-negative, case-control study across 14 hospitals in England finds that the…

On Nov. 12, 2025, UK researchers reported a test-negative, case-control study across 14 hospitals in England finds that the…

On Oct. 9, 2025, Boehringer Ingelheim’s JASCAYD® (nerandomilast) tablets has been approved by the U.S. Food and Drug…
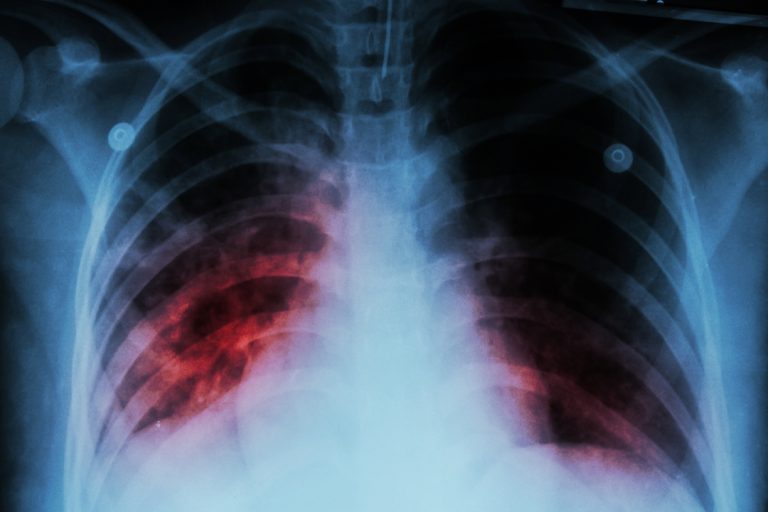
On May 15, 2025, the Multnomah County Health Department in Oregon reports an individual at Lane Middle School…

On Nov. 17, 2021, Pacific Northwest National Laboratory (PNNL) announced researchers had compiled the most comprehensive road map…