Cartoon Illustrating “The NIH and U.S. Healthcare in Crisis” Published
On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…

On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…

On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…
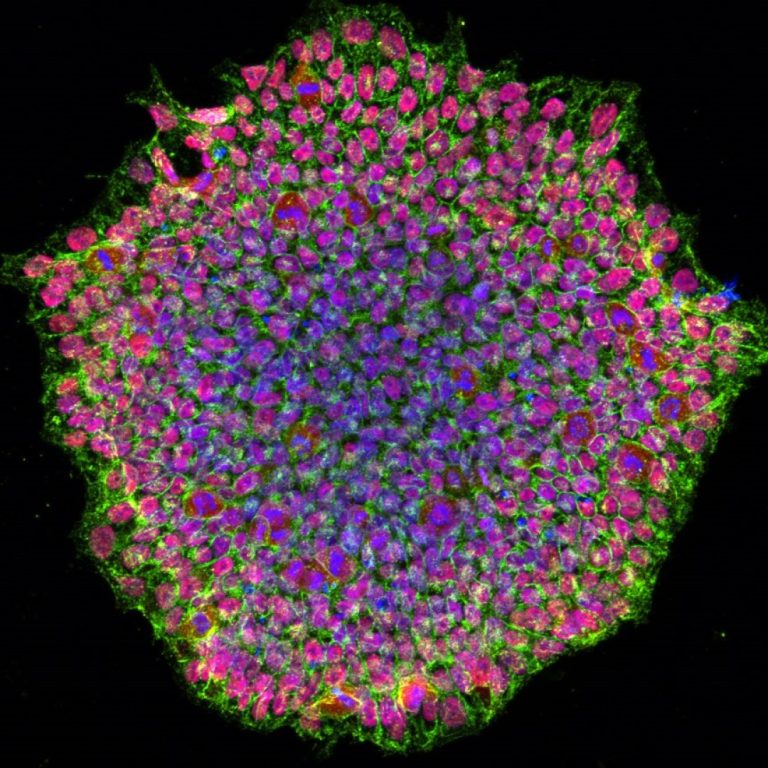
On Mar. 24, 2025, Hideyuki Okano, a stem-cell scientist at Keio University in Tokyo, and his colleagues announced…

On Jan. 16, 2025, Shionogi Inc. has been awarded a $375 million project agreement through the Rapid Response…

On Jan. 2, 2025, SIGA Technologies announced that its antiviral treatment TEPOXX (tecovirimat 200 mg capsules), marketed as…

On Dec. 17, 2024, scientists from Hiroshima University announced they had mapped near-gap-free and near-error-free genomes of a…
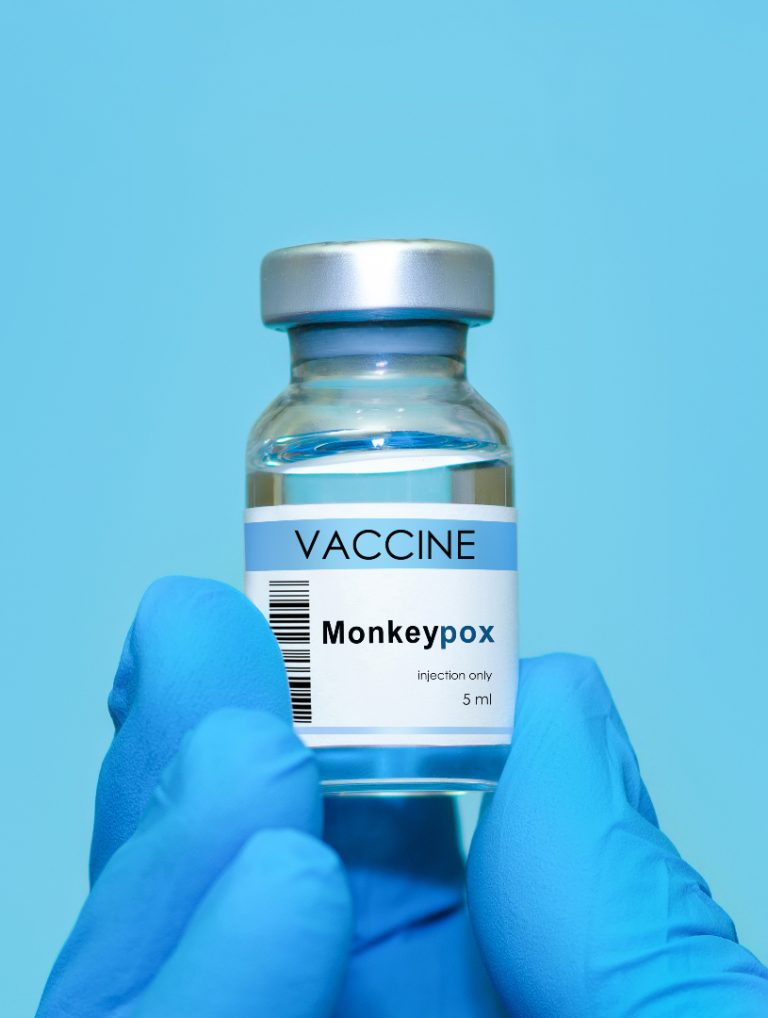
On Nov. 19, 2024, the World Health Organization (WHO) announced it had granted Emergency Use Listing (EUL) to Japan-based…
On Nov. 6, 2024, data from the VENUS (Vaccine Effectiveness, Networking, and Universal Safety) study in Japan revealed…

On Oct. 2, 2024, a team of researchers from the University of Tokyo announced they had discovered living…

On Sept. 13, 2024, CSL Seqirus and sa-mRNA pioneer Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor…
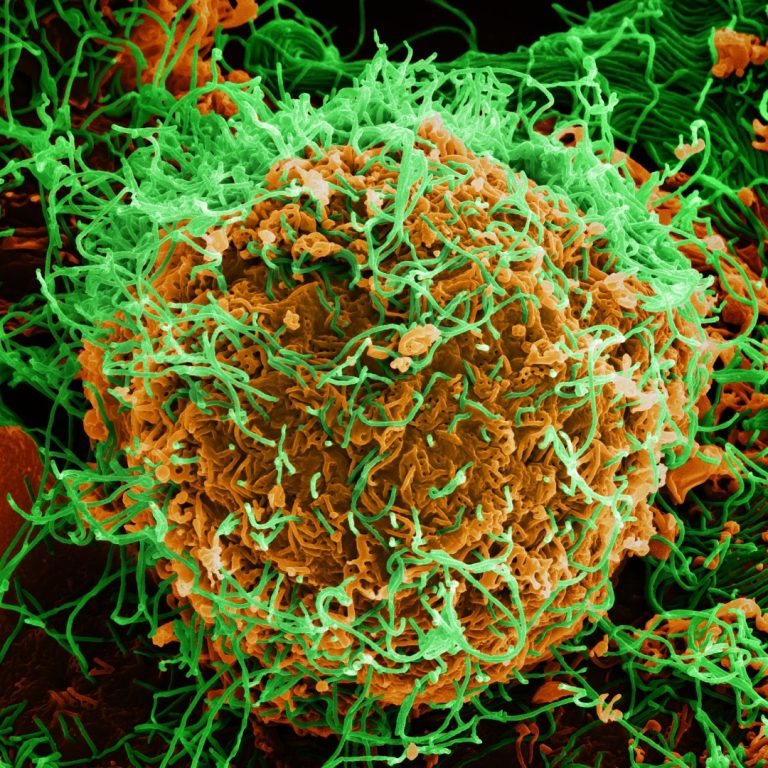
On Aug. 29, 2024, a small team of scientists has launched an open-source database for some of the…

On Jul. 3, 2024, Regeneron Pharmaceuticals and Sanofi announced that the European Commission (EC) has approved Dupixent® (dupilumab)…

On May 10, 2024, Novavax announced that it had entered into a co-exclusive licensing agreement with Sanofi. The…

On Feb. 16, 2024, Regeneron and Sanofi announced that the Ministry of Health, Labor and Welfare (MHLW) in…
On Feb. 5, 2024, CSL and and Arcturus Therapeutics announced the results of a follow-up analysis of a…

On Feb. 5, 2024, a U.S. Centers for Disease Control and Prevention (CDC) celebrated the opening of the…

On Dec. 21, 2023, CSL and and Arcturus Therapeutics announced the results of a Phase 3 study showing…

On Nov. 28, 2023, CSL and Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor and Welfare (MHLW)…

On Nov. 10, 2022, researchers from the Okinawa Institute of Science and Technology (OIST), in collaboration with K….

On Oct. 31, 2022, Moderna announced that it had received approval from the Ministry of Health, Labour and…

On Oct. 6, 2022, researchers in Japan released a study that described the development and application of the…
On Oct. 4, 2022, Washington University in St. Louis announced it had licensed the rights to develop, manufacture…

On Jul. 26, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had received expanded manufacturing and marketing approval…

On May 31, 2022, Moderna and Takeda announced the transfer of the marketing authorization for Moderna’s COVID-19 vaccine…

On Apr. 19, 2022, Novavax announced that its partner, Takeda, received manufacturing and marketing approval from the Japan…

On Mar. 16, 2022, Moderna announced an agreement with the Ministry of Health, Labour and Welfare of Japan…

On Jan. 18, 2022, Merck and Ridgeback Biotherapeutics announced the signing of a long-term supply agreement with the…

On Dec. 24, 2021, Merck and Ridgeback Biotherapeutics announced that Japanメs Ministry of Health, Labor and Welfare had…

On Dec. 15, 2021, Novavax announced the submission of a New Drug Application to the Ministry of Health,…

On Sept. 7, 2021, Takeda Pharmaceutical announced the Government of Japan’s Ministry of Health, Labour and Welfare purchase…