CIRM invested $10 million to build California Cell and Gene Therapy Manufacturing Network
On May 31, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $10 million to five facilities as…

On May 31, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $10 million to five facilities as…
On May 19, 2023, the U.S. Food and Drug Administration (FDA) approved Krystal Biotech’s Vyjuvek, a herpes-simplex virus…

On Apr. 5, 2023, researchers at Oregon Health & Science University’s OHSU Casey Eye Institute announced that gene…
On Jan. 4, 2023, an international research group announced it had for the first time reconstructed ancestors dating…

On Dec. 16, 2022, the U.S. Food and Drug Administration approved Ferring Pharmaceuticals’ Adstiladrin (nadofaragene firadenovec-vncg), a non-replicating…

On Nov. 22, 2022, the U.S. Food and Drug Administration approved the investigational gene therapy etranacogene dezaparvovec or…
On Aug. 24, 2022, BioMarin Pharmaceutical announced that the European Commission (EC) had granted conditional marketing authorization (CMA)…

On Aug. 17, 2022, the U.S. Food and Drug Administration approved Zynteglo (betibeglogene autotemcel) developed by bluebird bio,…

On Oct. 18, 2021, Athenex and the Center for Cell and Gene Therapy at Baylor College of Medicine,…

On Aug. 27, 2021, National Aeronautics and Space Administration (NASA) Space station astronauts successfully demonstrated DNA repair in…

On May 10, 2021, the National Institutes of Health reported that an investigational gene therapy can safely restore…

On Mar. 27, 2021, the FDA approved Abecma (idecabtagene vicleucel), a cell-based gene therapy to treat adult patients…
On Feb. 17, 2021, Novartis announced that it had entered into a grant agreement with the Bill &…

On Feb. 5, 2021, the FDA approved Breyanzi (lisocabtagene maraleucel), a cell-based gene therapy to treat adult patients…

On Jan. 28, 2021, scientists at the National Eye Institute (NEI) have developed a promising gene therapy strategy…

On Nov. 9, 2020, Ultragenyx announced that it planned to build a new large-scale gene therapy manufacturing facility…

On Oct. 22, 2020, a newly developed light-sensing protein called the MCO1 opsin restored vision in blind mice…

On Aug. 24, 2020, Catalent announced that it had contracted to provide drug substance manufacturing to AstraZeneca for…
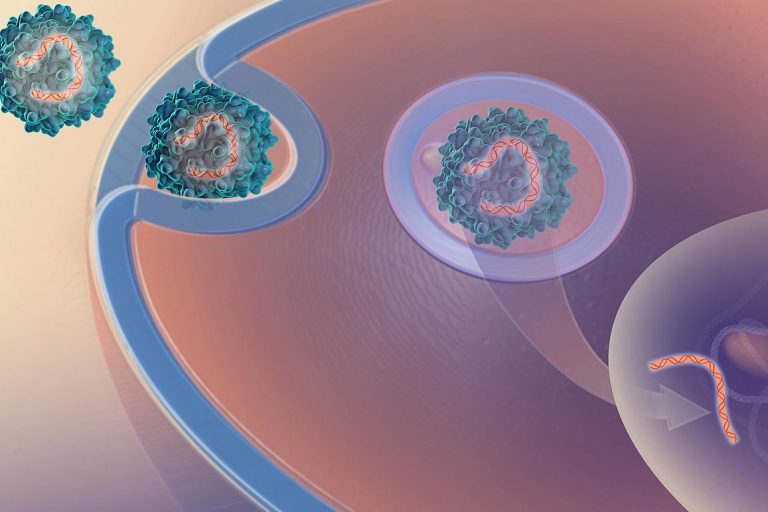
On Oct. 7, 2019, Penn Medicine announced it had developed gene therapy to treat Duchenne muscular dystrophy (DMD)…

On Apr. 18, 2019, St. Jude announced a cure for SCID-X1, commonly known as bubble boy disease. By…

On Mar. 13, 2019, Nature published a call for a global moratorium on heritable human genome editing signed by…

On Jun. 7, 2018, the Wold Foundation of Wyoming announced a gift of $2.5 million to help in…

On Aug. 30, 2017, the U.S. Food and Drug Administration (FDA) approved Kymriah (tisagenlecleucel) for certain pediatric and…

On Mar. 5, 2014, Oregon Health & Science University (OHSU) announced the creation of the Center for Embryonic…

On Aug. 24, 2007, BioLife Solutions was incorporated in Seattle. BioLife Solutions (formerly Cryomedical Sciences) is a class-defining…

On Sept. 30, 2003, University of Iowa (UI) Microbiology Professor Mark Stinski made the discoveries of CMV promoter…
In 2003, China granted the world’s first regulatory approval of a gene therapy product, Gendicine (Shenzhen SiBiono GenTech),…

In 2002, Stanford geneticist Mark Kay uses a gene-therapy technique known as RNA inhibition to switch off genes…

On Jan. 11, 2001, Oregon Health Sciences University (OHSU) researchers reported the production of the world’s first genetically…

On Oct. 25, 1996, the first gene therapy trial for patients with Hunter syndrome was begun at the…