Vaxart provided update on its oral COVID-19 vaccine program
On Mar. 31, 2020, Vaxart announced it had produced five COVID-19 vaccine candidates for testing in its preclinical…

On Mar. 31, 2020, Vaxart announced it had produced five COVID-19 vaccine candidates for testing in its preclinical…

On Mar. 31, 2020, a team led by Pawel Kalinski, MD, PhD, of Roswell Park Comprehensive Cancer Center…
On Mar. 30, 2020, the partners in the COVID-19 Therapeutics Accelerator announced grants of $20 million to three…

On Mar. 30, 2020, Humanigen announced that the company had submitted an initial protocol synopsis to the FDA…

On Mar. 28, 2020, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to…

On Mar. 27, 2020, Humanigen announced that the company had submitted an initial protocol synopsis to U.S. Food…

On Mar. 27, 2020, Emory’s Vaccine and Treatment Evaluation Unit (VTEU) announced participation in a clinical trial testing…
On Mar. 26, 2020, physician-scientists at four University of California Health medical centers — UC San Diego Health,…

On Mar. 25, 2020, employees of Sartorius Stedim Biotech in Aubagne, France are keeping production running to ensure…

On Mar. 24, 2020, the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern announced it had joined…

On Mar. 23, 2020, researchers from the University of Oxford the launch of a new clinical trial to…

On Mar. 20, 2020, Humanigen announced that the company was seeking to conduct a Phase III, randomized, controlled,…

On Mar. 20, 2020, Novartis announced its commitment to donate up to 130 million doses of generic hydroxychloroquine…

On Mar. 19, 2020, Roche announced it was working with the U.S. Food & Drug Administration (FDA) to…

On Mar. 19, 2020, iBio announced its progress towards developing vaccine candidates for preventing infection from the SARS-CoV-2…

On Mar. 16, 2020, the National Institutes of Health (NIH announced that a Phase 1 clinical trial evaluating…
On Mar. 15, 2020, Fosun Pharma and BioNTech announced a strategic development and commercialization collaboration to advance BioNTech’s…

On Mar. 13, 2020, Johnson & Johnson announced that its Janssen Pharmaceutical Companies have entered a collaboration with…

On Mar. 12, 2020, Emory University announced it was participating in a National Institutes of Health (NIH) sponsored…
On Mar. 9, 2020, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the…

On Mar. 2, 2020, Ophirex, a public-benefit biotechnology company working to improve outcomes for global victims of snakebite,…
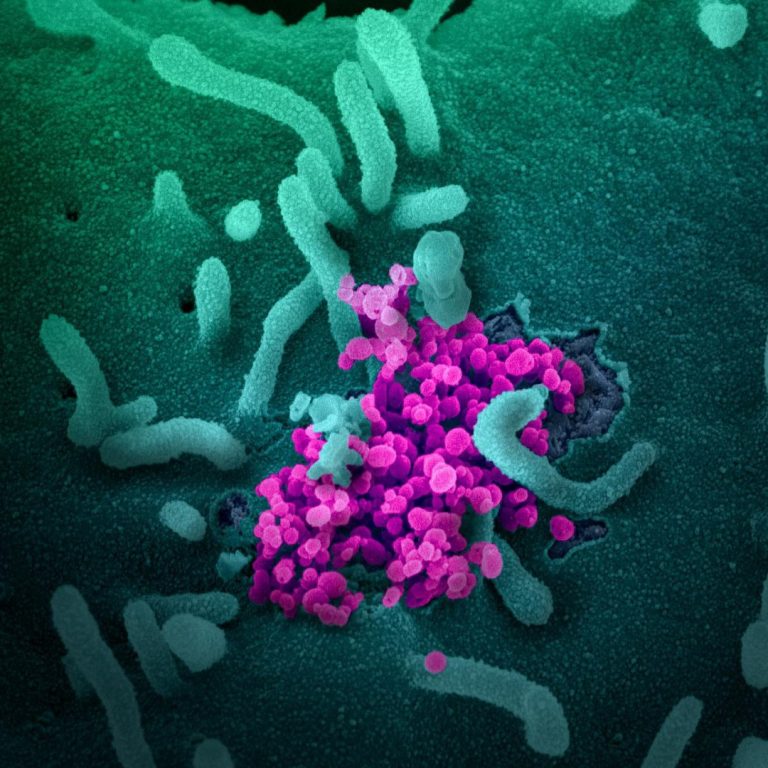
On Feb. 25, 2020, the National Institutes of Health (NIH) announced that a randomized, controlled clinical trial to…
On Feb. 25, 2020, a University of British Columbia (UBC) researcher was part of an international team working…

On Feb. 24, 2020, Innovation Pharmaannounced the Company submitted a Material Transfer Agreement (MTA) with a leading U.S.-based…

On Feb. 22, 2020, a federal judge from the Southern District of New York ruled drug companies, device…

On Feb. 21, 2020, Lundbeck announced that VYEPTI™ (eptinezumab-jjmr) had been approved by the U.S. Food and Drug…

On Feb. 18, 2020, Innovation Pharma announced the Company was exploring its lead defensin mimetic drug candidate, Brilacidin,…

On Feb. 16, 2020, Medigen Vaccine Biologics (MVB) and the National Institutes of Health (NIH) announced they had…
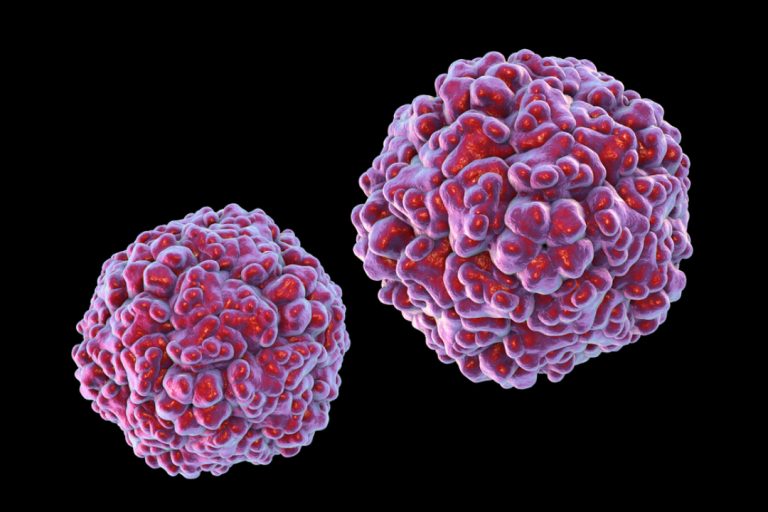
On Dec. 25, 2019, Medigen Vaccine Biologics (MVB) announced it had completed the enrollment of phase III multi-region…
On Dec. 20, 2019, Vaccitech’s partner, the University of Oxford’s Jenner Institute, in collaboration with The King Abdullah…