INOVIO announced initiation of phase 2/3 clinical trial for COVID-19 DNA vaccine candidate, INO-4800
On Nov. 16, 2020, INOVIO announced that it had received clearance from the the U.S. Food & Drug…

On Nov. 16, 2020, INOVIO announced that it had received clearance from the the U.S. Food & Drug…

On Nov. 16, 2020, Biological E. Limited (BE), a Hyderabad-based vaccines and pharmaceutical company, Dynavax Technologies, and Baylor…

On Nov. 16, 2020, Innovation Pharmaceuticals announced that an overseas Clinical Trial Application (CTA) has been submitted to…

On Nov.12, 2020, the governing Board of the California Institute for Regenerative Medicine (CIRM) approved four new clinical…
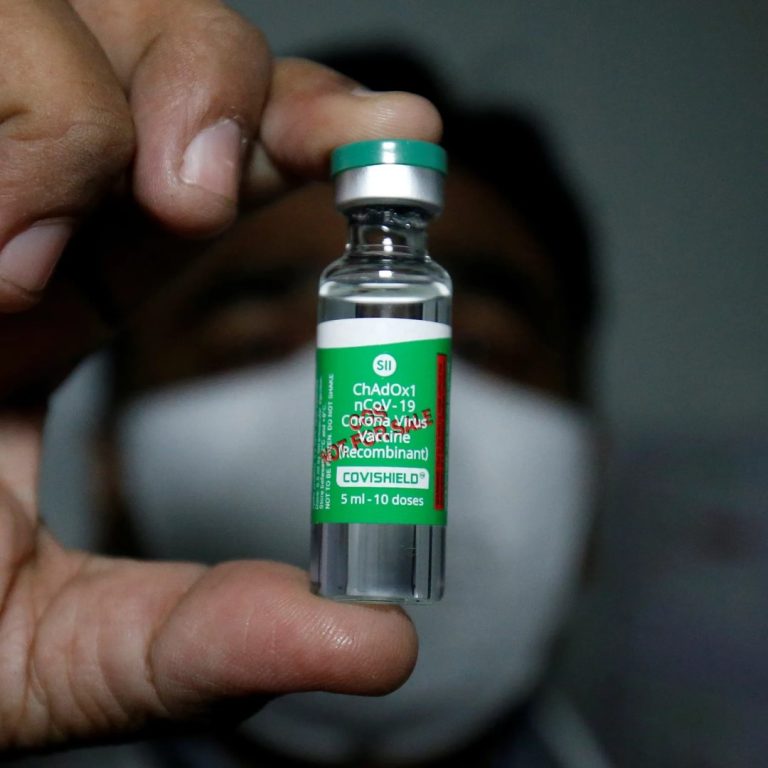
On Nov. 11, 2020, the Serum Institute of India (SII) and Indian Council of Medical Research (ICMR) announced…
On Nov. 9, 2020, the NIH reported that a pre-exposure prophylaxis (PrEP) regimen containing an investigational long-acting form…
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…

On Nov. 9, 2020, a study published in JAMA reported the effect of hydroxychloroquine on clinical status at…

On Nov. 6, 2020, Humanigen announced that it had entered into a Cooperative Research and Development Agreement (CRADA)…

On Nov. 4, 2020, Mateon Therapeutics announced the receipt of approval from Instituto Nacional de Salud (INS), the…

On Nov. 3, 2020, Medigen Vaccine Biologics (MVC) and the National Institute of Hygiene and Epidemiology (NIHE), a…

On Nov. 2, 2020, Innovation Pharma announced receipt of written feedback from the FDA that is in general…

On Oct. 30, 2020, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced enrollment of volunteers in a phase…

On Oct. 30, 2020, Humanigen announced that MedStar Washington Hospital Center in Washington, D.C. treated its first COVID-19…

On Oct. 30, 2020, Icosavax announced the launch of the companyメs COVID-19 vaccine program with preclinical data on…

On Oct. 29, 2020, Takeda Pharmaceutical announced that it would import and distribute 50 million doses of Moderna’s…

On Oct. 23, 2020, the U.S. FDA authorised a restart of clinical trials of the ChAdOx1 nCov-2019 Oxford…

On Oct. 23, 2020, AstraZeneca announced that the clinical trials for the AstraZeneca Oxford coronavirus vaccine, AZD1222, had…

On Oct. 22, 2020, Roche and Atea Pharmaceuticals announced they had joined forces in the fight against COVID-19…

On Oct. 22, 2020, a newly developed light-sensing protein called the MCO1 opsin restored vision in blind mice…
On Oct. 21, 2020, results from an international, randomized, controlled clinical trial indicated that a four-month daily treatment…

On Oct. 21, 2020, ImmunityBio and NantKwest announced that the first patient had been dosed in the Phase…

On Oct. 19, 2020, Mateon Therapeutics announced the receipt of approval from Republica Argentina Poder Ejecutivo Nacional, the…

On Oct. 19, 2020, LabCorp announced a test that provided a quantitative measurement of an individualメs SARS-CoV-2 IgG…

On Oct. 16, 2020, the NIH launched an adaptive Phase 3 clinical trial to evaluate the safety and…

On Oct. 15, 2020, ImmunityBio announced it had received authorization from the FDA to begin a Phase I…

On Oct. 14, 2020, Sorrento Therapeutics announced receipt of clearance from the Brazilian regulatory agency (ANVISA) to proceed…

On Oct. 14, 2020, Vaxart announced that the FDA had completed its review of the Companyメs Investigational New…
On Oct. 13, 2020, Humanigen announced that the National Institute of Allergy and Infectious Diseases (NIAID), had launched…

On Oct. 13, 2020, the National Institute of Allergy and Infectious Diseases (NIAID), launched a study designed to…