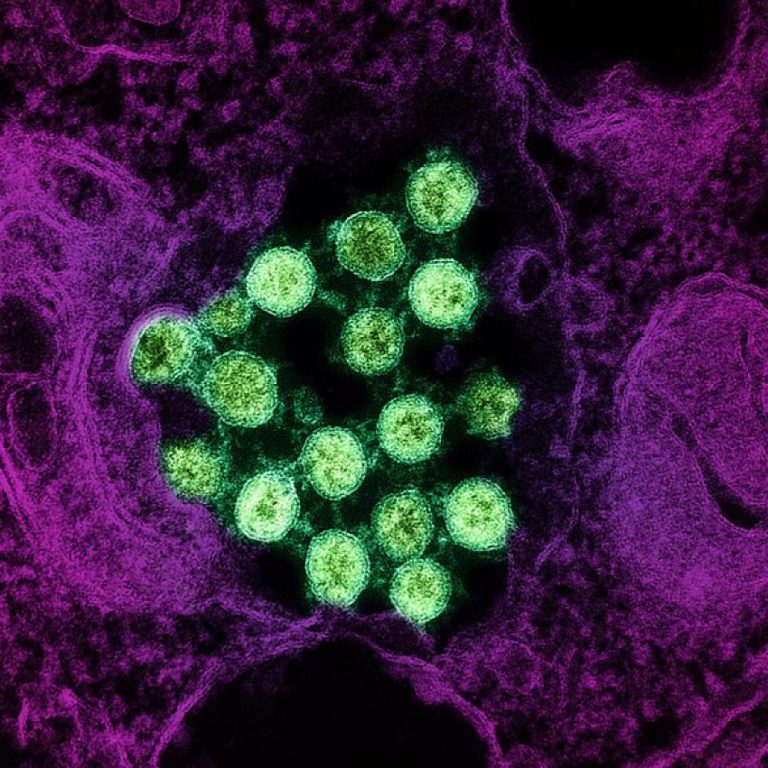
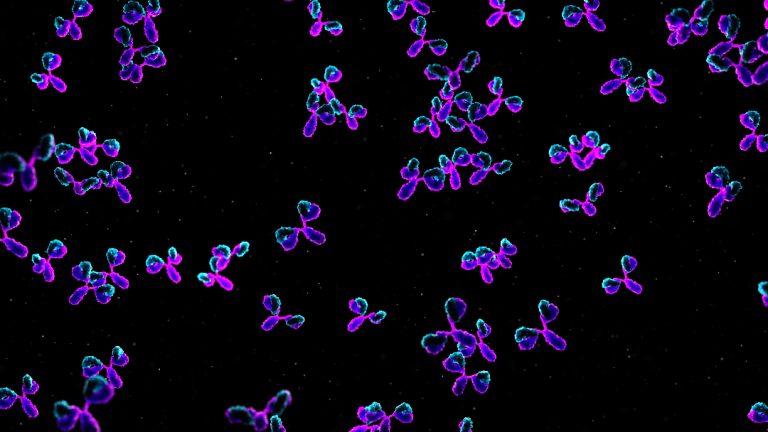
Sorrento Therapeutics and ViralClear entered agreement to explore combination antibody plus antiviral therapy against COVID-19
On Sept. 30, 2020, ViralClear Pharmaceuticals and Sorrento Therapeutics announced the companies were exploring the synergistic potential of…