Ohio Department of Health Confirmed Multiple cases in Spreading Measles Outbreak
On Mar. 25, 2025, Ohio Department of Health (ODH) announced a measles outbreak in Ashtabula County and one…

On Mar. 25, 2025, Ohio Department of Health (ODH) announced a measles outbreak in Ashtabula County and one…

On Mar. 25. 2025, the UK Health Security Agency (UKHSA) published its view on the pathogen families that…

On Mar. 25, 2025, the Oklahoma State Department of Health (OSDH) reported that nine additional measles cases had…

On Mar. 24, 2025, As bird flu ravages poultry farms across the country – including in South Dakota…

On Mar. 24, 2025, Tuberculosis (TB) infections among children in the European region rose 10% in 2023, indicating…

On Mar. 21, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…
On Mar. 21 , 2025, the Tennessee Department of Health confirmed the state’s first measles case in 2025…
On Mar. 20, 2025, Ohio Department of Health (ODH) reported the state’s first measles case of 2025. The…

On Mar. 20, 2025, the U.S. Department of Agriculture (USDA) in consultation with the U.S. Department of Health…

On Mar. 17, 2025, The Mississippi Board of Animal Health (MBAH) has been notified by the U.S.Department of…

On Mar. 14, 2025, the Michigan Department of Health and Human Services (MDHHS) and Oakland County Health Division…
On Mar. 14, 2025, Sanofi announced the immediate adoption of influenza strains selected by the U.S. Food and…

On Mar. 14, 2025, the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) announced an…

On Mar. 14, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…

On Mar. 14, 2025, the Oklahoma State Department of Health (OSDH) received notification of two more probable measles…

On Mar. 14, 2025, researchers from Cornell University reported results from a study that show the Aging Process…

On Mar. 13, 2025, the U.S. Food and Drug Administration (FDA) announced recommendations to vaccine manufacturers for the…

Mar. 13. 2025, scientists who examined the impact of an H5N1 avian flu outbreak in an Ohio dairy…

On Mar. 13, 2025, The Kansas Department of Health and Environment (KDHE) and the Stevens County Health Department…

On Mar. 12, 2025, the Animal and Plant Health Inspection Service (APHIS) confirmed the presence of highly pathogenic…

On Mar. 11, 2025, the New York State Department of Health today announced the first case of measles…

On Mar. 11, 2025, researchers from Texas A&M University School of Public Health report findings show significant links…

On Mar. 11, 2025, in an excerpt from his book Booster Shots (Penguin Random House), pediatrician and infectious…

On Mar. 11, 2025, the Oklahoma State Department of Health (OSDH) reported the first two measles cases in…

On Mar. 8, 2025, the World Health Organization (WHO) announced that since the outbreak of Sudan virus disease…

On Mar. 7, 2025, the Texas Department of State Health Services reported updated information about an outbreak of…

On Mar. 6, 2025, the New Mexico Department of Health (NMDOH) confirms that a deceased resident of Lea…
On Mar. 5, 2025, the World Health Organization (WHO) announced In the past two decades, tuberculosis (TB) prevention,…
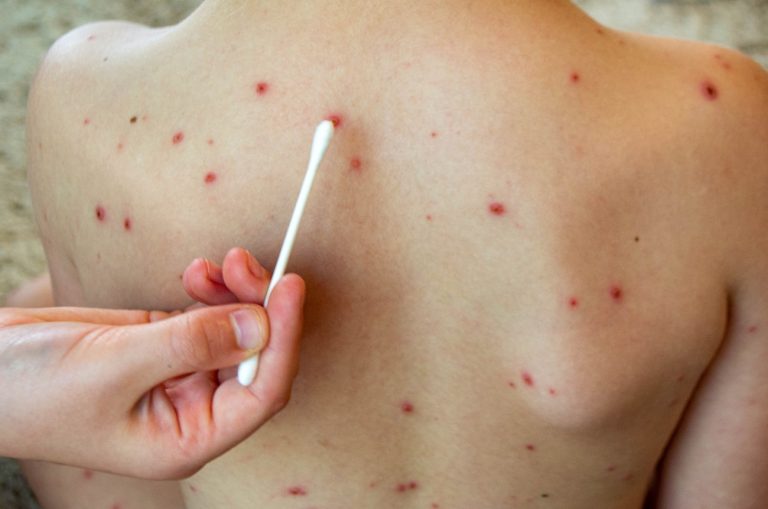
On Mar. 4, 2025, the Texas Department of State Health Services reported an outbreak of measles in the…

On Mar. 3, 2025, LifeScienceHistory.com announced the release of “Earth is a Rock, Let the DNA Mold Me”…