Diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed was licensed
On Jul. 31, 1996, the diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (Tripedia by Aventis Pasteur)…
On Jul. 31, 1996, the diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (Tripedia by Aventis Pasteur)…

On Jun. 18, 1996, the International AIDS Vaccine Initiative (IAVI) was launched, calling for the speedy development of…

On Mar. 29, 1996, the second inactivated hepatitis A vaccine (Vaqta by Merck) was licensed.

In 1996, A/goose/Guangdong/1/1996 (H5N1), the precursor of the highly pathogenic H5N1 avian influenza viruses (HPAIVs) was identified in…
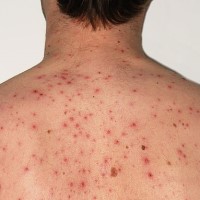
On Mar. 17, 1995, the varicella virus vaccine, live (Varivax by Merck) was licensed for the active immunization…

On Feb. 22, 1995, the first inactivated hepatitis A vaccine, distributed by SmithKline Beecham Pharmaceuticals (Philadelphia, Pennsylvania), was…

In 1995, researchers led by Dr. Peter St. George-Hyslop at the University of Toronto discovered and cloned two…

In 1995, Hollister-Stier became Bayer Pharmaceuticals. Bayer had acquired Cutter in 1974 and Miles in 1978. Hollister-Stier Laboratories,…

In 1995, the Immunization Practices Advisory Committee (ACIP), American Academy of Pediatrics, and the American Association of Family…
On Nov. 28, 1994, the typhoid Vi polysaccharide inactivated injectable polysaccharide vaccine (Typhim Vi by Aventis Pasteur) was…

On Oct. 1, 1994, the U.S. Department of Health and Human Services (HHS) implemented the Vaccines for Children…
On Sept. 29, 1994, based on recommendations of the national certification committees and after review of surveillance and…
On Aug. 20, 1994, the entire Western Hemisphere was certified as “polio-free” by the International Commission for the…
In March 1994, the Global Programme for Vaccines and Immunization was created, merging two World Health Organization (WHO)…

On Dec. 1, 1993, scientists reported the recovery of DNA unique to Mycobacterium tuberculosis from a lung lesion…

On Aug. 10, 1993, In response to this measles epidemic, the U.S. Congress created the National Vaccine Program…

On Mar. 30, 1993, the U.S. Food and Drug Administration (FDA) approved a new Haemophilus b conjugate vaccine,…

In 1993, The Institute of Medicine published “The Children’s Vaccine Initiative: Achieving the Vision.” The Children’s Vaccine Initiative…

In 1993, the development of immunization registries was promoted at the national level. A national health goal for…

On Aug. 10, 1993, The Vaccines for Children Program was established after passage of the U.S. Congress’ Omnibus…

In 1993, the U.S. Centers for Disease Control and Prevention (CDC) responded to the H5N1 avian flu outbreak…
On Dec. 10, 1992, the Japanese encephalitis (JE) virus vaccine inactivated (JE-Vax by Research Foundation for Microbial Diseases…
On Aug. 21, 1992, the Food and Drug Administration (FDA) approved licensure of a second DTaP product, prepared…

On Dec. 17, 1991, the Diphtheria and tetanus toxoids and acellular pertussis vaccine (Acel-Imune by Lederle) was licensed…

On Nov. 22, 1991, the Immunization Practices Advisory Committee (ACIP) published recommendations for routine hepatitis B vaccination for…
On Jan. 11, 1991, recommendations of Immunization Practices Advisory Committee (ACIP) for routine Hib vaccination for infants beginning…
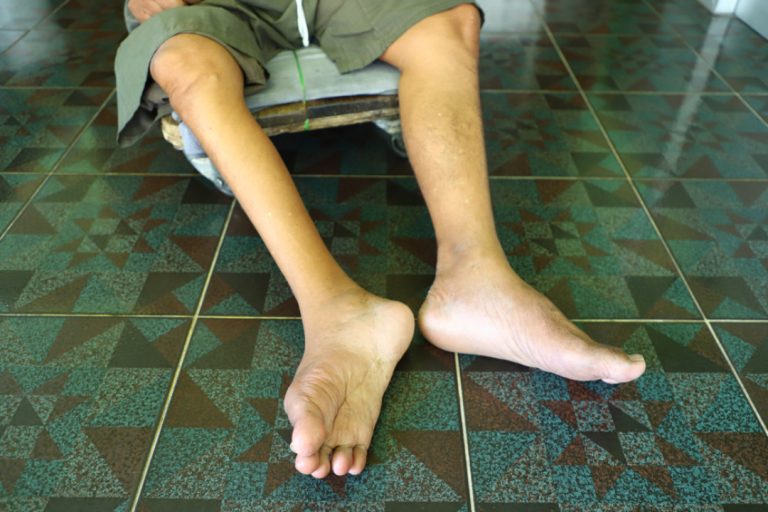
In 1991, the last case of indigenous polio in the Western Hemisphere occurred in a 5-year-old boy, Luis…

In 1991, The Northwestern University Cancer Center was dedicated through an endowment gift from Ann and Robert H….
On Dec. 13, 1990, the U.S. Food and Drug Administration (FDA) approved Haemophilus b Conjugate Vaccine (Meningococcal Protein…

On Apr. 13, 1990, the Immunization Practices Advisory Committee (ACIP) recommendations for use of any of the three…