Arizona Department of Agriculture reports Avian Influenza Detected at commercial farm
On May 20, 2025, the Arizona Department of Agriculture (AZDA) reported that poultry at a commercial farm located…

On May 20, 2025, the Arizona Department of Agriculture (AZDA) reported that poultry at a commercial farm located…

On May 20, 2025, member States of the World Health Organization (WHO) formally adopted by consensus the world’s…

On May 20, 2025, The Trump administration said it will limit approval for seasonal COVID-19 shots to seniors…

On May, 20, 2025, a team of scientists has developed a fresh approach: the first mRNA bird-flu vaccine…
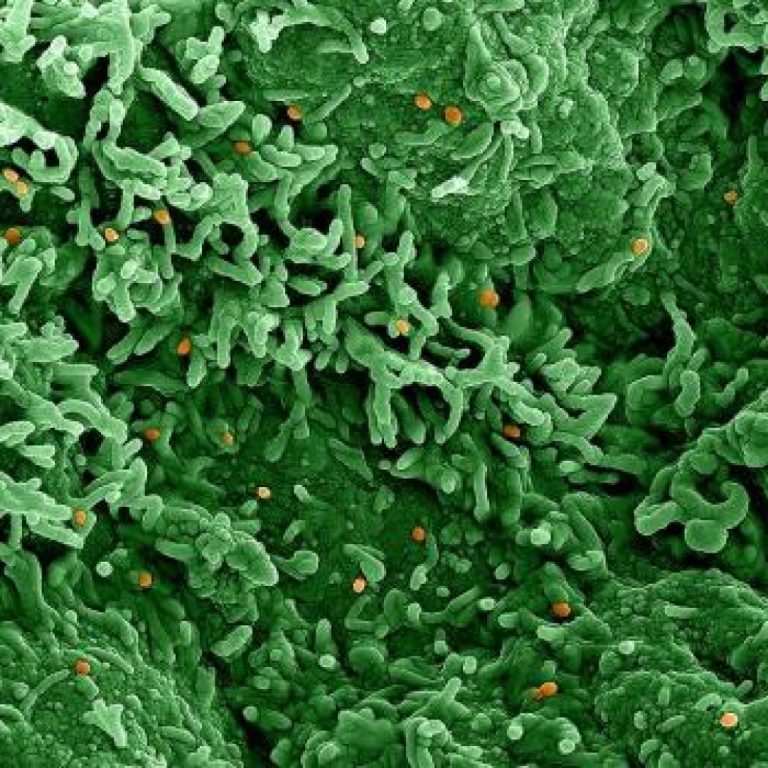
On May 19, 2025, Scripps scientists, in collaboration with researchers in Nigeria and Cameroon, announced genomic data has…

On May 17, 2025, The U.S. Food and Drug Administration approved Novavax’s COVID-19 vaccine, but placed additional conditions…

On May 16, 2025, the Texas Department of State Health Services (DSHS) reported 718 cases of measles have…

On May 15, 2025, the World Health Organization (WHO) announced it has published its World health statistics report…

On May 15, 2025, the New Mexico Department of Health (NMDOH) confirmed the first measles cases in Sandoval…

On May 13, 2025, Seattle & King County was notified of a confirmed measles case in a Canadian…

On May 9, 2025, The World Health Organization (WHO) and Medicines Patent Pool (MPP) announced a sublicensing agreement…
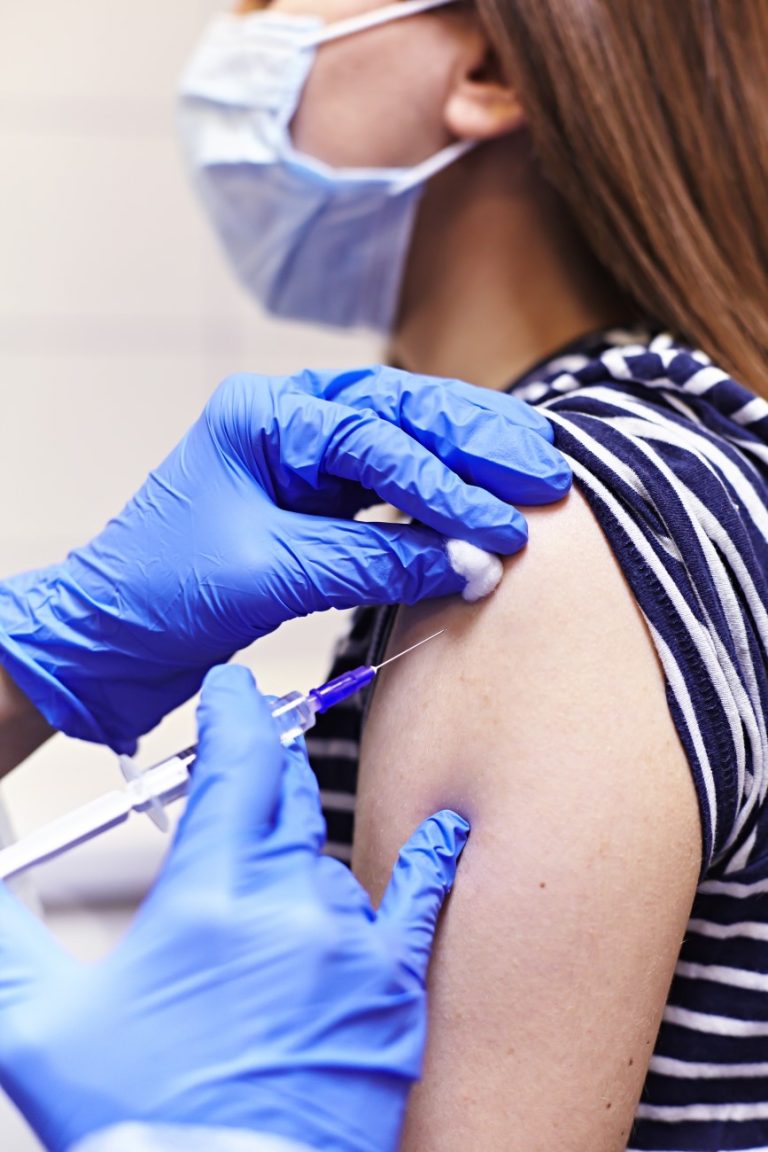
On May 9, 2025, the Texas Department of State Health Services (DSHS) reported 709 cases of measles have…

On May 8, 2025, Bill Gates announced that he has decided to give away his money back to…

Om My 8, 202, Merck Animal Health and Kansas Governor Laura Kelly jointly announced today the $895 million…
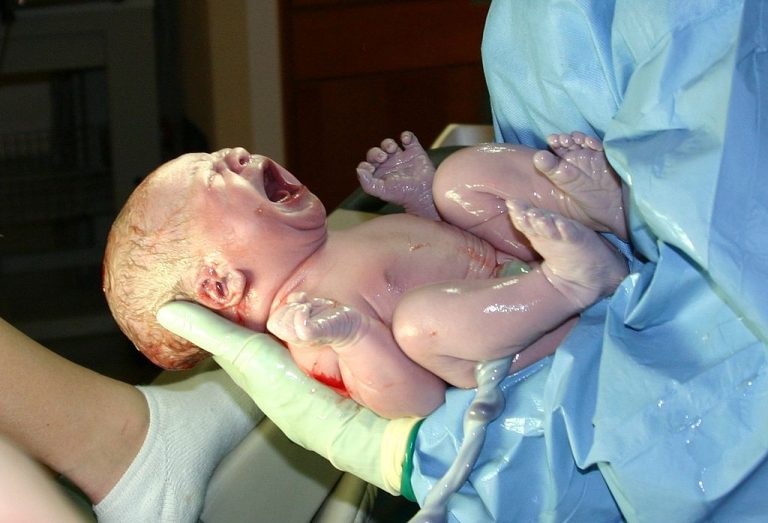
On May 7, 2025, the New Mexico Department of Health (NMDOH) confirmed the first measles cases the first…

On May 7, 2025, researchers at the University College London (UCL) and King’s College London announced that an…

On May 5, 2025, The European Union (EU) and France announced half a billion euros worth of incentives…

On May 2, 2025, the Louisiana Department of Health (LDH) reported an increase in whooping cough (pertussis) cases…

On May 2, 2025, the Centers for Disease Control reported twelve pediatric deaths associated with seasonal influenza virus…

On May 2, 2025, the Texas Department of State Health Services (DSHS) reported 683 cases of measles have…

On May 1, 2025, the U.S. National Institutes of Health (NIH), the world’s largest funder of biomedical research,…
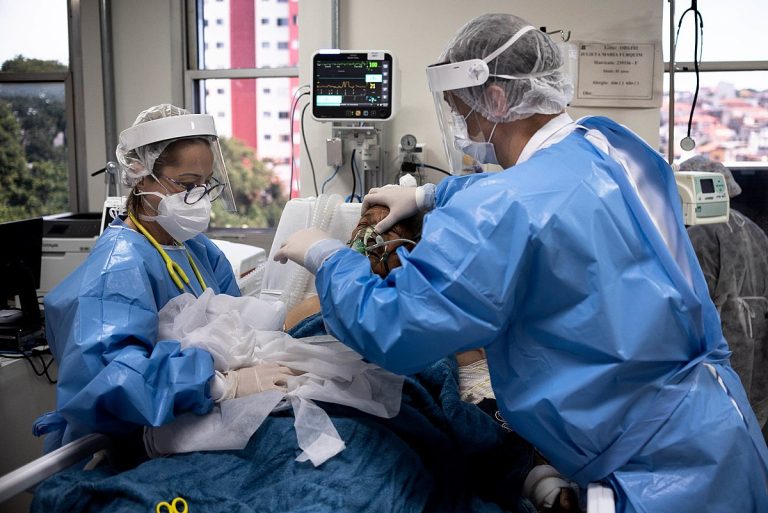
On May 1, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced that hospitals are no…

On Apr. 29, 2025, BioMADE announced Maple Grove as the Minnesota site in its national network of bioindustrial…

On Apr. 28, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced it had awarded a more than $3…
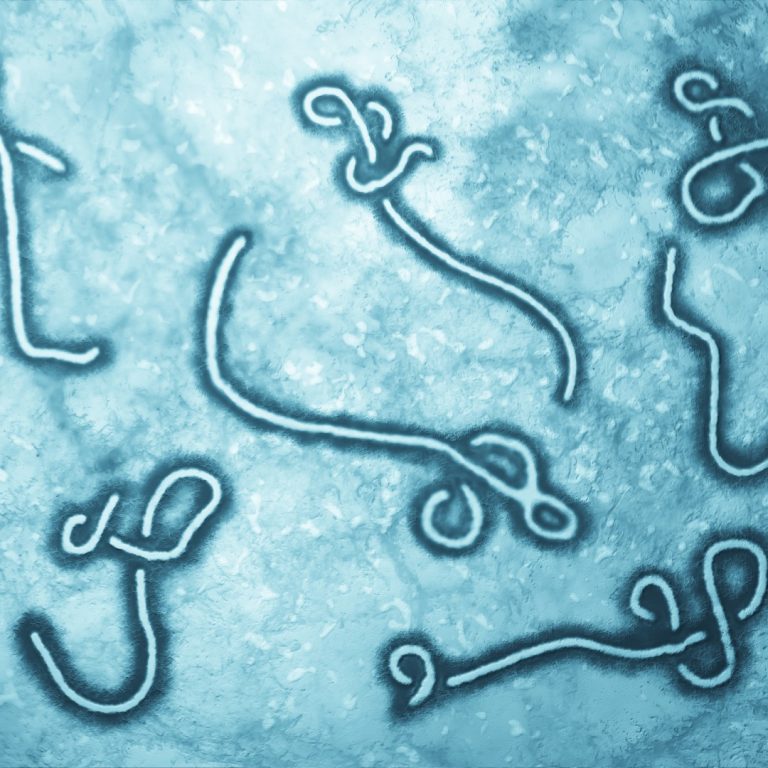
On Apr. 26, 2025, the World Health Organization (WHO) reported that Uganda has declared the end of the…
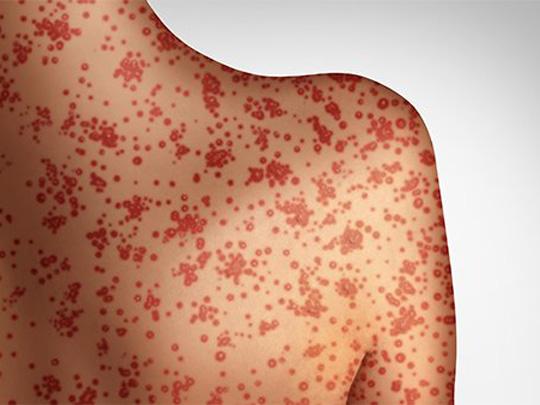
On Apr. 25, 2025, the Texas Department of State Health Services (DSHS) reported that 646 cases of measles…
On Apr. 22, 2025, Seattle & King County was notified of a confirmed measles case in a King…

On Apr. 22, 2025, Syngenta announced that its next-generation insecticide Sovrenta® has received pre-qualification by World Health Organization…

On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…

On Apr. 19, 2025, the Louisiana Department of Health (LDH) confirmed one case of measles in an adult…