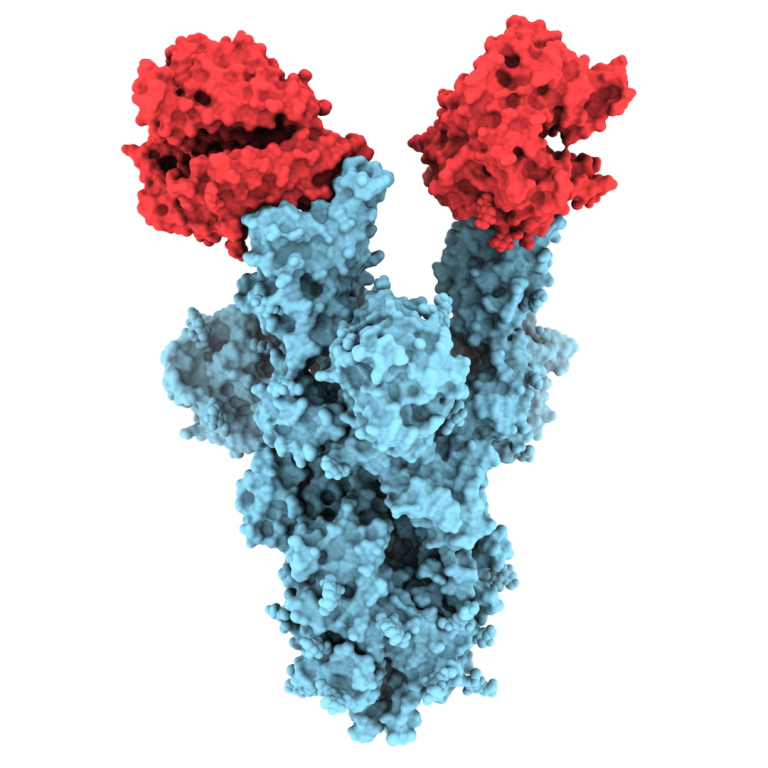
IVI, INOVIO, and KNIH partner with CEPI in phase 1/2 clinical trial of INOVIO’s COVID-19 DNA vaccine in South Korea
On Apr. 16, 2020, the International Vaccine Institute (IVI) announced that the Coalition for Epidemic Preparedness Innovations (CEPI)…












![Dynavax donated 10,000 doses of HEPLISAV-Bᆴ [Hepatitis B Vaccine (Recombinant), adjuvanted] vaccine to emergency healthcare providers](https://lifesciencehistory.com/wp-content/uploads/2024/05/dynavax_logo-3.png)