California Department of Public Health Reported Single, Possible Case of Bird Flu Virus in Child with Mild Symptoms
On Nov. 19, 2024, the California Department of Public Health (CDPH) reported it has identified a possible bird…

On Nov. 19, 2024, the California Department of Public Health (CDPH) reported it has identified a possible bird…
On Nov. 19, 2024, the World Health Organization (WHO) announced it had granted Emergency Use Listing (EUL) to Japan-based…
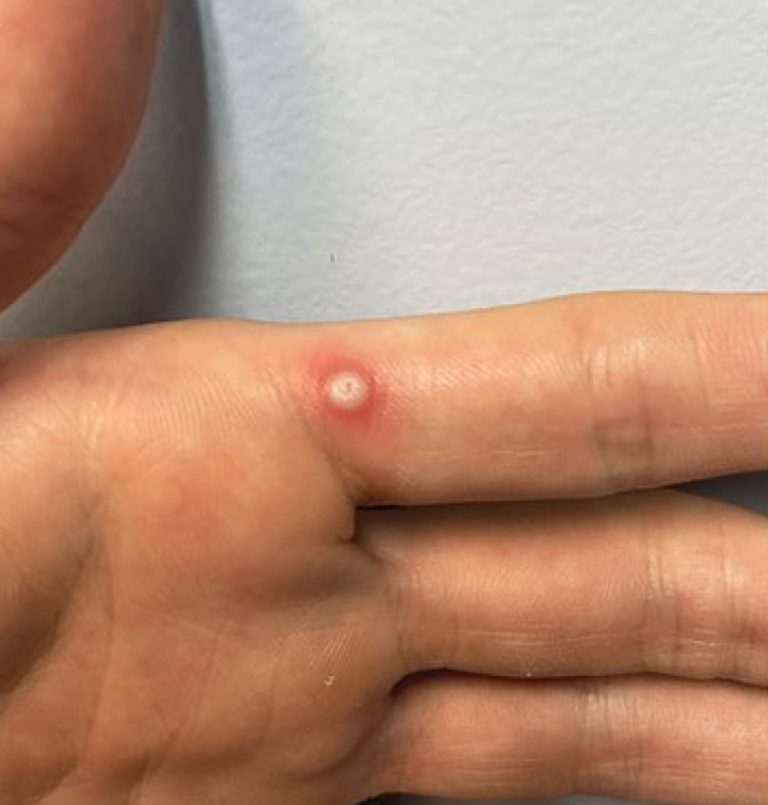
On Nov. 16, 2024, the California Department of Public Health (CDPH) announced it had identified through laboratory testing…

On Nov. 15, 2024, the U.S. Centers for Disease Control and Prevention (CDC) confirmed a highly pathogenic avian…

On Nov. 15, 2024, The Hawai‘i Department of Agriculture (HDOA) received confirmation from the U.S. Department of Agriculture…

On Nov. 14, 2024, the March of Dimes released its 2024 Report Card, revealing the U.S. preterm birth…

On Nov. 14, 2024, the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention…

On Nov. 13, 2024, a study published in JAMA Network Open showed that respiratory syncytial virus (RSV) was…
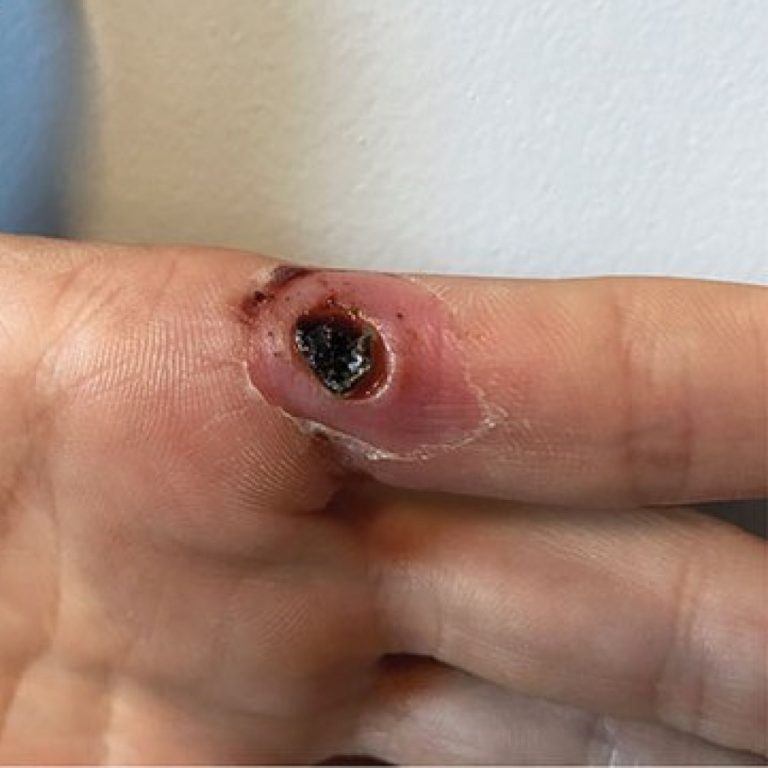
On Nov. 9, 2024, the World Health Organization announced that three additional countries had confirmed travel-related cases of…
On Nov. 9, 2024, the BC Centre for Disease Control’s Public-Health Laboratory reported that an an individual in…

On Nov. 8, 2024, Anixa Biosciences announced updated positive data from the Phase 1 clinical trial of its…
On Nov. 7, 2024, researchers reported that Implementation of routine immunization of U.S. adolescents with the quadrivalent (four-strain)…

On Nov. 6, 2024, the World Health Organization announced that the Access and Allocation Mechanism (AAM) for mpox…

On Nov. 6, 2024, researchers from the Pasteur Institute in Cambodia published a detailed genetic analysis of the…
On Nov. 6, 2024, data from the VENUS (Vaccine Effectiveness, Networking, and Universal Safety) study in Japan revealed…
On Nov. 6, 2024, researchers from Emory University, in collaboration with scientists at Gladstone Institutes and University of…

On Nov. 5, 2024, a World Health Organization (WHO) study published in eBioMedicine named 17 pathogens that regularly…
On Nov. 4, 2024, a team of University of Wisconsin–Madison researchers warned that artificial intelligence tools gaining popularity…

On Nov. 4, 2024, a study conducted by researchers from the CUNY Graduate School of Public Health and…

On Oct. 31, 2024, the Oregon Department of Agriculture reported that bird flu cases are on the rise,…

On Oct. 30, 2024, the the U.S. Department of Agriculture (USDA) reported that H5N1 bird flu was confirmed…
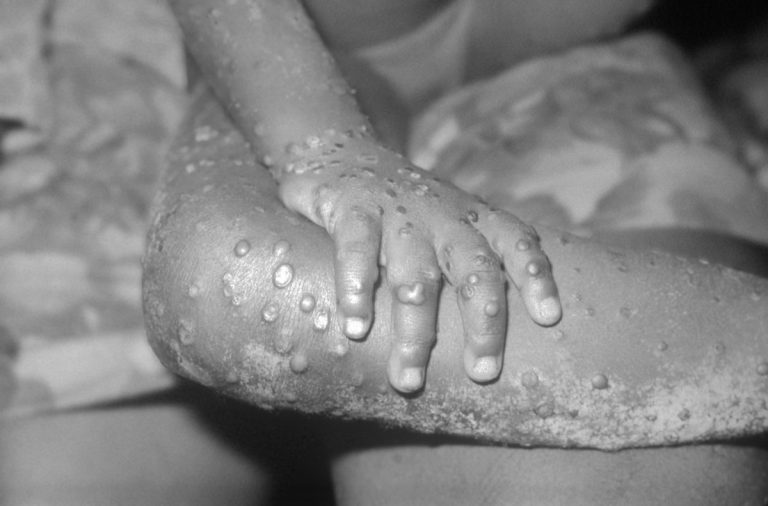
On Oct. 29, 2024, the World Health Organization (WHO) announced that it had, in collaboration with Member States,…
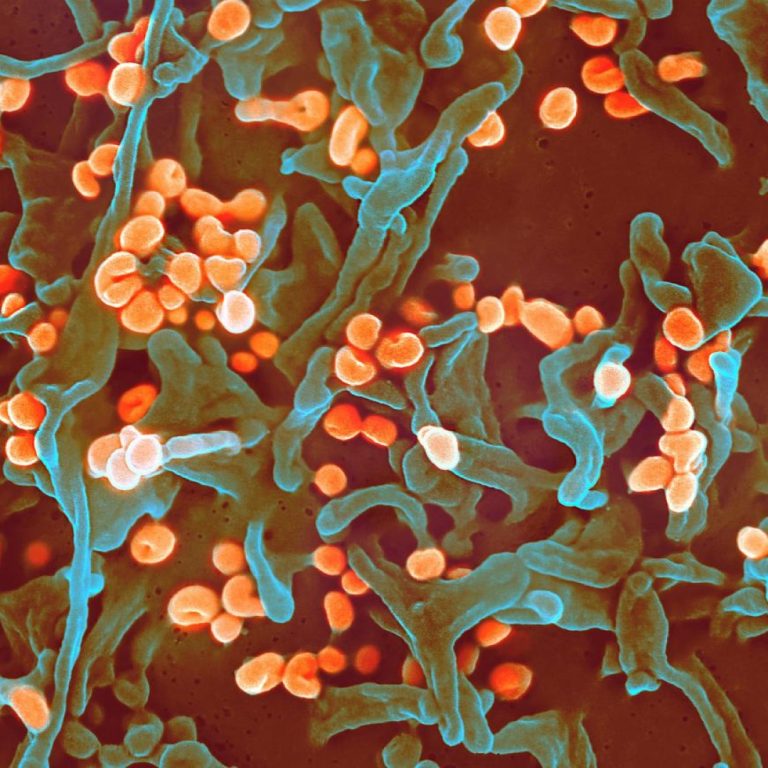
On Oct. 28, 2024, the Iowa Department of Health and Human Services (HHS) confirmed the death of a…
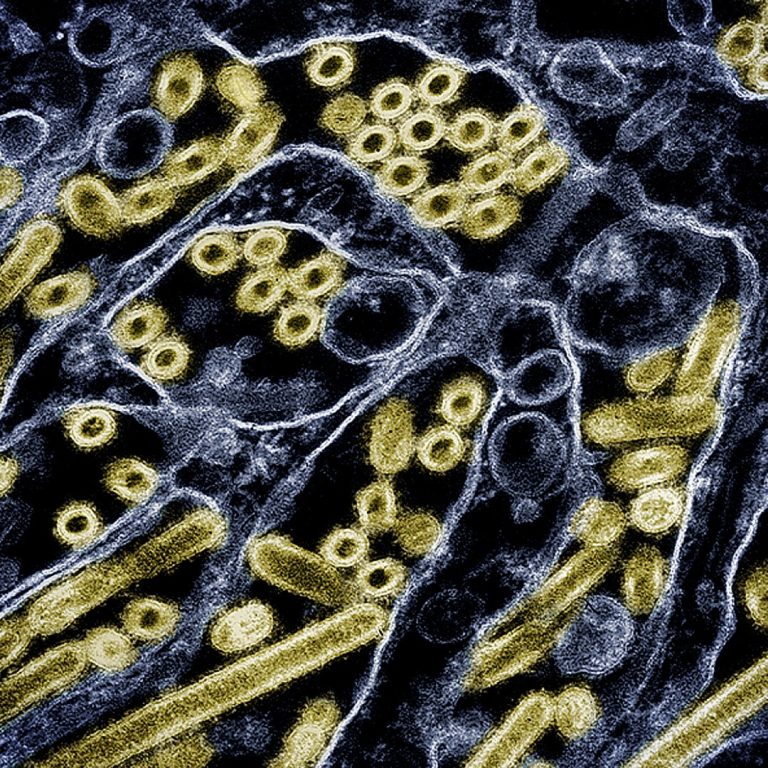
On Oct. 28, 2024, University of Wisconsin–Madison researchers reported that a strain of H5N1 avian influenza virus found…

On Oct. 24, 2024, the Centers for Disease Control and Prevention (CDC) confirmed that two people who had…

On Oct. 23, 2024, Pfizer announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…

On Oct. 22, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO® (Respiratory…
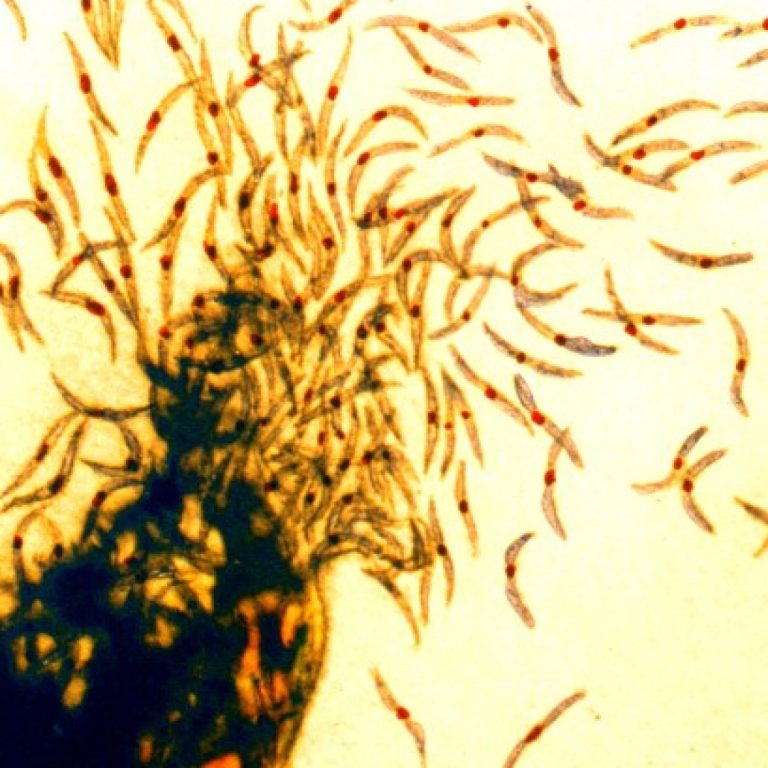
On Oct. 20, 2024, the World Health Organization (WHO) announced it had certified Egypt as malaria-free, marking a…

On Oct. 20, 2024, the Washington State Department of Health announced that four agricultural workers tested positive for…

On Oct. 18, 2024, the World Health Organization (WHO) reported that from January 1 to September 29, 2024,…