Arizona Department of Agriculture reported Influenza Detected in Dairy Cattle
On Feb. 11, 2025, the Arizona Department of Agriculture (AZDA) reported working in conjunction with the United States…

On Feb. 11, 2025, the Arizona Department of Agriculture (AZDA) reported working in conjunction with the United States…

On Feb. 5, 2025, the Spokane Regional Health District announced the first confirmed death from pertussis (whooping cough)…
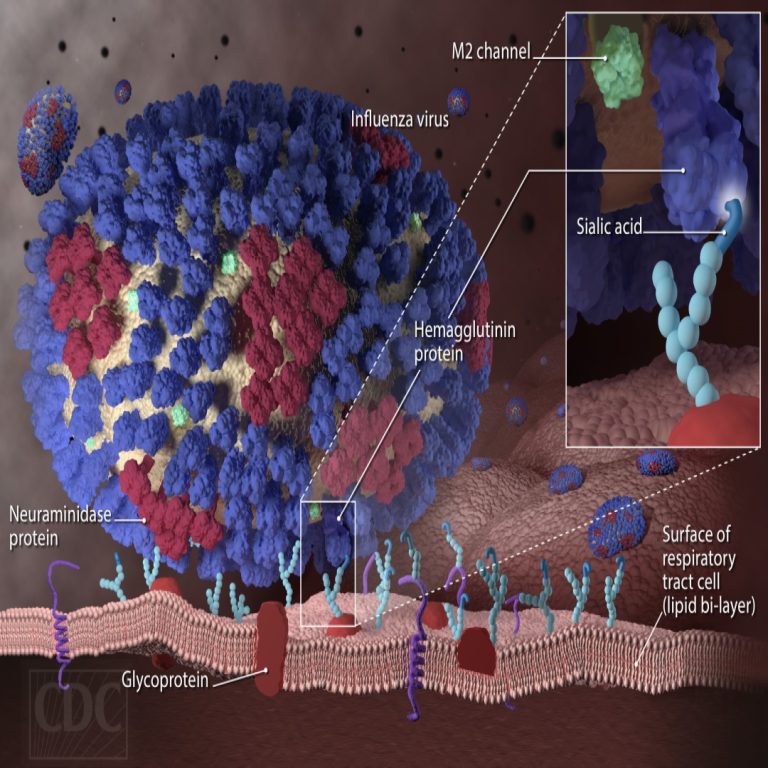
On Jan. 31, 2025, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Jan. 28, 2025, Rose Acre Farms, the nation’s second largest egg producer, reported that tests have confirmed…

On Jan. 27, 2024, the World Organisation for Animal Health (WOAH) reported that highly pathogenic avian influenza (HPAI)…
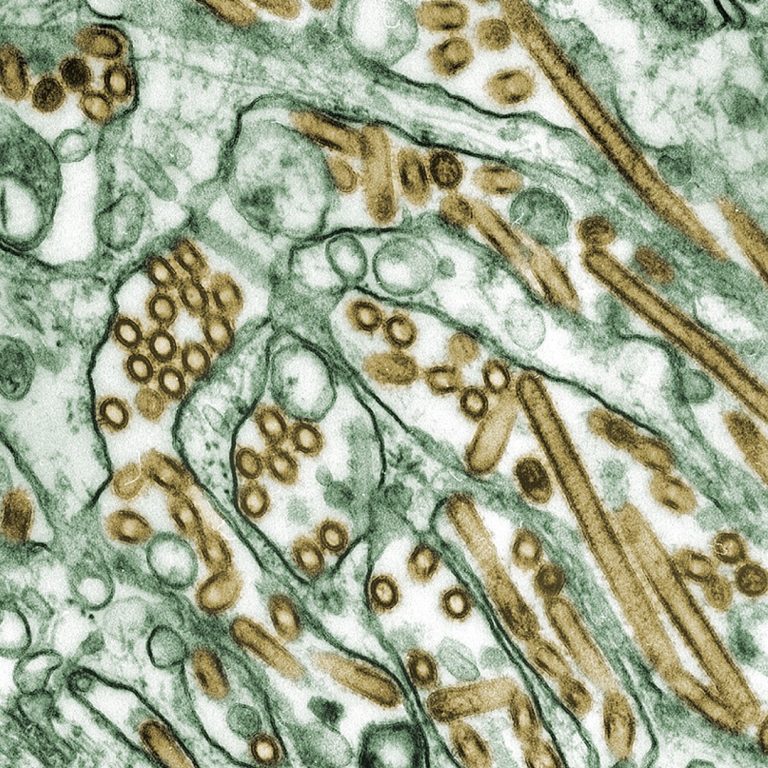
On Jan. 27, 2025, researchers reported that the H5N1 virus is adapting to new mammalian hosts, raising the…

Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…
On Jan. 24, 2025, the World Health Organization (WHO) reported that from January 1 to December, 29, 2024,…

On Jan. 24, 2025, researchers from the Veterans Affairs Portland Health Care System in Oregon reported results from…

On Jan. 24, 2025, The South Dakota Department of Health reported the death of a child due to…

On Jan. 22, 2025, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) has…

On Jan. 20, 2025, the White House announced the United States will exit the World Health Organization (WHO)….
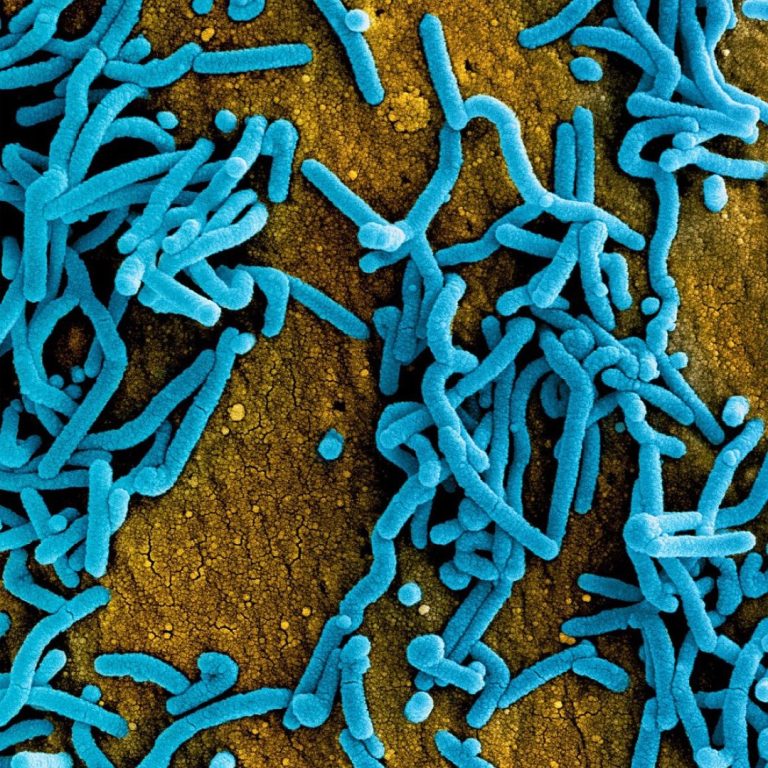
On Jan. 20, 2025, the World Health Organization (WHO) announced that Tanzania has confirmed an outbreak of Marburg…
On Jan. 20, 2025, a large international 68-country survey of 71,922 respondents found that in most countries, most…

On Jan. 17, 2025, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) reported that an…
On Jan. 17, 2025, The U.S. government announced it had awarded Moderna $590 million to advance the development…

On Jan. 17, 2025, the U.S. Centers for Disease Control and Prevention (CDC) reported that eleven pediatric deaths…

On Jan. 16, 2025, Shionogi Inc. has been awarded a $375 million project agreement through the Rapid Response…

On Jan. 15, 2025, officials from Lincoln Park Zoo in Chicago reported that testing had confirmed the highly pathogenic…

On Jan. 15, 2025, the U.S. Department of Commerce’s Bureau of Industry and Security (BIS) released an Interim…

On Jan. 15, 2025, the California Department of Public Health (CDPH) reported thatqOne confirmed human infection with influenza…
On Jan. 14, 2025, The U.S. Department of Defense (DoD) has awarded up to $11.9 million to Oregon…

On Jan. 13, 2025, the Texas Animal Health Commission (TAHC) and the United States Department of Agriculture’s (USDA)…

On Jan. 13, 2025, the World Health Organization (WHO) informed its Member States and IHR State Parties of…

On Jan. 10, 2025, the Institute for Health Metrics and Evaluation (IHME) reported that when it comes to…
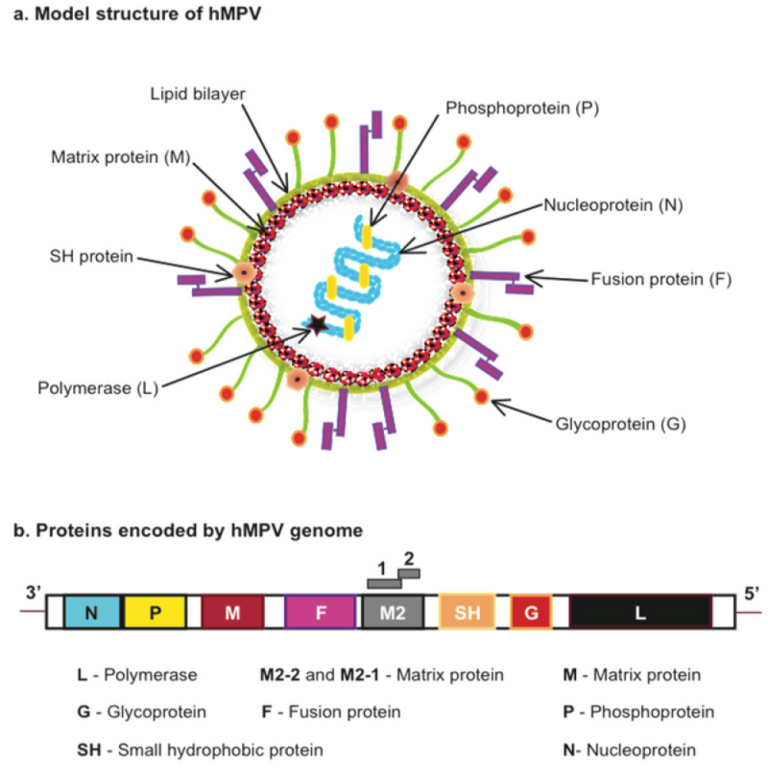
On Jan. 7, 2025, the World Health Organization (WHO) reported that trends in acute respiratory infections (ARI) in…
On Jan. 6, 2025, the Louisiana Department of Health reports the patient who had been hospitalized with the…

On Jan. 3, 2025, the California Department of Public Health (CDPH) reported that Whooping cough (pertussis) activity in…

On Jan. 3, 2025, the Centers for Disease Control and Prevention (CDC) reported that during Christmas week, respiratory…

On Jan. 3, 2025, the U.S. Department of Health and Human Services (HHS) announced it would award $306…