Pioneering cancer plasticity atlas will help predict response to cancer therapies
On Jun. 12, 2025, the Wellcome Sanger Institute, Parse Biosciences and the Computational Health Center at Helmholtz Munich…

On Jun. 12, 2025, the Wellcome Sanger Institute, Parse Biosciences and the Computational Health Center at Helmholtz Munich…
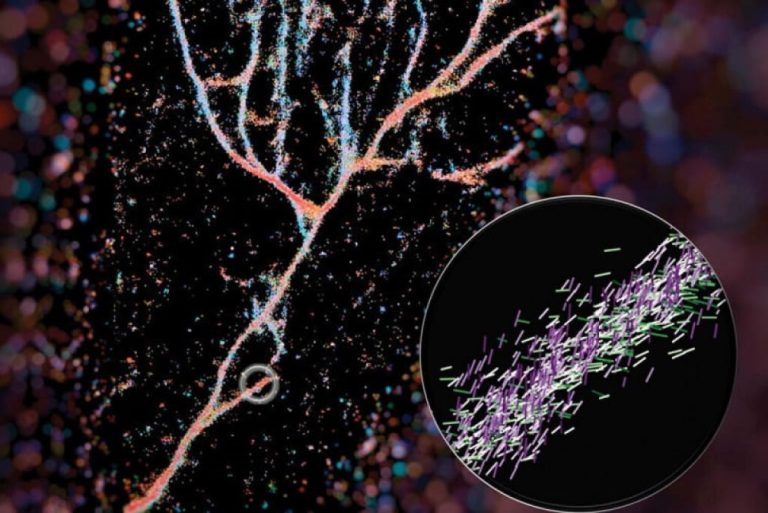
On Jun. 11, 2025, researchers from the Wellcome Sanger Institute, Centre of Genomic Regulation (CRG) and Institute for…

On Jun. 11, 2025, a study led by by University of Barcelona experts Carles J. Ciudad and Verònica…

On Jun. 11, 2025, researchers at the University of California, Davis, announced they have developed an investigational brain-computer…
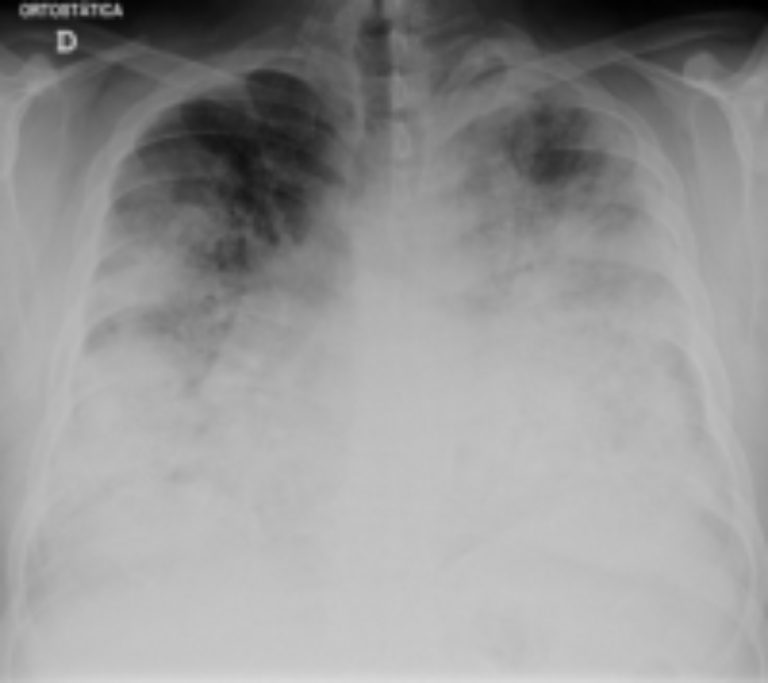
On Jun. 10, 2025, two patients treated within the past two months at the University of Washington Medical…

On Jun. 9, 2025, Oakland County Health Division in Michigan is notifying the public about a measles exposure…
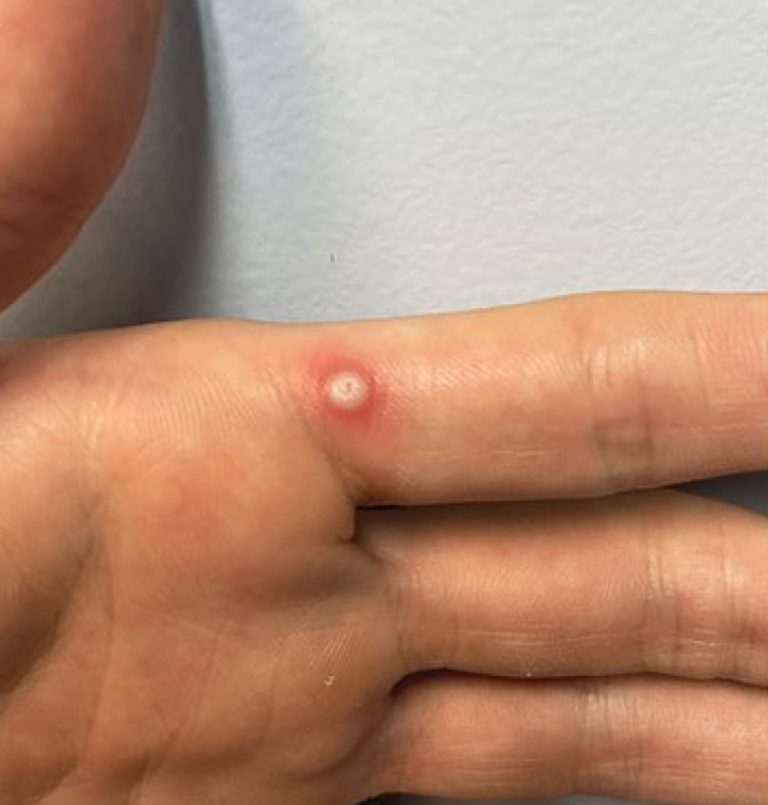
On Jun. 9, 2025, the World Health Organization (WHO) said the mpox outbreak is still a public health…
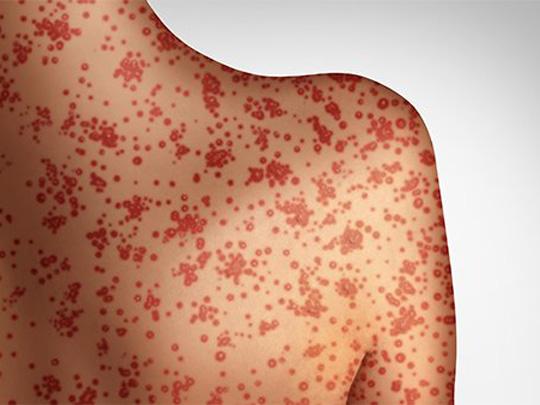
On Jun. 6, 2025, the South Dakota Department of Health has notified the public of potential measles exposure…

On Jun. 5, 2025, research from University of Southern California, Dornsife (USC Dornsife) scientists reveals how cells fix…

On Jun. 4, 2025, the Center for Scientific Integrity — the non-profit organization that runs Retraction Watch —…

On Jun. 4, 2025, the Conference of State Parties (CoSP) of the African Medicines Agency (AMA) announced the…

On Jun. 3, 2025, the U.S. Army Combat Capabilities Development Command Chemical Biological Center, DEVCOM CBC, and the…

On Jun. 2, 2025, the South Dakota Department of Health reported the first measles case of 2025 in…

On Jun. 2, 2025, Sanofi and Blueprint Medicines, a US-based, publicly traded biopharmaceutical company specializing in systemic mastocytosis…

On Jun. 2, 2025, the U.S. Food and Drug Administration (FDA) announces it has launched Elsa, a generative…

On May 30, 2025, Pfizer announced statistically significant and clinically meaningful survival results from the Phase 3 BREAKWATER…
On May 29, 2025, The California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On May 27, 2025, Eli Lilly and SiteOne Therapeutics, a private biotechnology company developing small molecule inhibitors of…
On May, 27, 2025, an Ohio State University (OSU) study has revealed the biological secret to the Zika…

On May 22, 2025, Gladstone scientists unveiled a powerful computation tool that integrates different forms of biological data…

On May 20, 2025, the World Health Organization (WHO) announced that world leaders pledged at least an additional…

On May 19, 2025, Prime Medicine announced positive initial data from the first patient dosed in its ongoing…

On May 19, 2025, researchers at the Broad Institute Ben Neale and Mark Daly are co-leading the study,…

On May 19, 2025, Regeneron Pharmaceuticals announced it has been named the successful bidder in the bankruptcy auction…

On May 19, 2025, researchers from the Karlsruhe Institute of Technology (KIT) announced they have have discovered how…

On May 16, 2025, the U.S. Food and Drug Administration has cleared Fujirebio Diagnostics’ blood test to diagnose…

On May 15, 2025, a University of Colorado–led case report in Emerging Infectious Diseases, describes a 58-year-old woman…
On May 15, 2025, Amneal Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) has approved Brekiya®…

On May 15, 2025, the World Health Organization (WHO) announced it has published its World health statistics report…

On May 15, 2025, a team at Children’s Hospital of Philadelphia (CHOP) and Penn Medicine announced that a…