Mayo Clinic research confirmed critical role of masks in preventing COVID-19 infection
On Nov. 24, 2020, published data from researchers at Mayo Clinic found that physical separation reduced the exposure…

On Nov. 24, 2020, published data from researchers at Mayo Clinic found that physical separation reduced the exposure…

On Nov. 23, 2020, the U.S. Food and Drug Administration (FDA) expanded the approved indication for Genentech’s Xofluza…

On Nov. 23, 2020, CytoDyn announced it had reached enrollment of 293 patients in its Phase 3 trial…
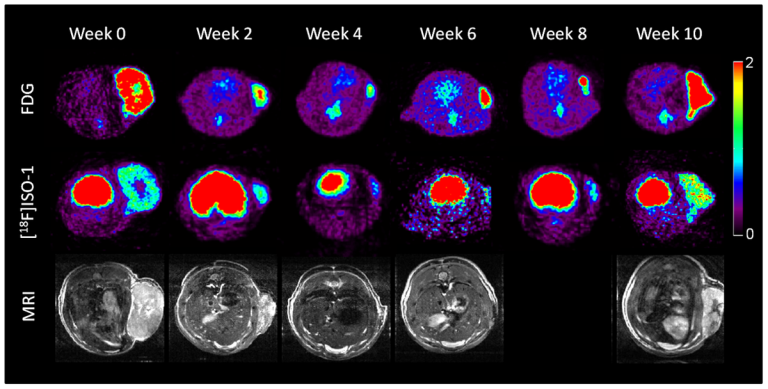
On Nov. 19, 2020, in a comprehensive analysis of patients with cancer who had exceptional responses to therapy,…
On Nov. 19, 2020, Novartis announced that it had entered into an exclusive worldwide license and collaboration agreement…
On Nov. 19, 2020, Medigen Vaccine Biologics announced that preclinical results of their COVID-19 vaccine candidate had been…

On Nov. 19, 2020, Eli Lilly and Incyte announced that the FDA had issued an Emergency Use Authorization…
On Nov. 19, 2020, XBiotech announced data for its breakthrough candidate therapy for treating infections of influenza and…

On Nov. 19, 2020, Dallas-based company, Worlds Inc., the U.S. Air Force and Texas A&M University announced a…

On Nov. 18, 2020, Gilead Sciences announced topline results from the Phase 2/3 CAPELLA trial evaluating the company’s…

On Nov. 17, 2020, RedHill Biopharma announced that the U.S. Food and Drug Administration (FDA) had cleared the…

On Nov. 17, 2020, the Mayo Clinic reported that more than 900 employees had contracted COVID-19 in the…

On Nov. 16, 2020, RedHill Biopharma announced that the U.S. Phase 2 study with opaganib (Yeliva, ABC294640)1 in…

On Nov. 16, 2020, the UK Department for Health and Social Care Testing Innovation Fund announced £12.2M funding…

On Nov. 13, 2020, Roche announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Nov. 13, 2020, Merck announced that the U.S. Food and Drug Administration (FDA) had approved KEYTRUDA, Merck’s…
On Nov. 13, 2020, NeuroRx and Relief Therapeutics announced that to-date, 150 patients (out of a targeted enrollment…
On Nov. 13, 2020, Agilent Technologies announced it had received U.S. Food and Drug Administration (FDA) approval for…

On Nov. 13, 2020, Moderna announced that Swissmedic had started a rolling review of mRNA-1273, the Company’s vaccine…

On Nov. 12, 2020, AstraZeneca announced that the CALAVI Phase II trials for Calquence (acalabrutinib) in patients hospitalised…
On Nov. 11, 2020, Sorrento Therapeutics announced that it was filing an investigational new drug application (IND) for…

On Nov. 11, 2020, Mesoblast announced that the randomized controlled Phase 3 trial of remestemcel-L in patients with…

On Nov. 11, 2020, Mateon Therapeutics announced an agreement with Windlas Biotech of India to commercialize ARTIVeda. ARTIVeda…

On Nov. 19, 2020, Medigen Vaccine Biologics and BlueWillow Biologics announced a partnership to develop S-2P-NE-01, a nasal…

On Nov. 9, 2020, Ultragenyx announced that it planned to build a new large-scale gene therapy manufacturing facility…
On Nov. 9, 2020, Pfizer and BioNTech announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 had demonstrated evidence…
On Nov. 9, 2020, the NIH reported that a pre-exposure prophylaxis (PrEP) regimen containing an investigational long-acting form…
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…

On Nov. 9, 2020, a study published in JAMA reported the effect of hydroxychloroquine on clinical status at…

On Nov. 6, 2020, Humanigen announced that it had entered into a Cooperative Research and Development Agreement (CRADA)…