Oragenics SARS-CoV-2 spike protein produced neutralizing antibodies with Intramuscular and intranasal adjuvants
On Aug. 30, 2021, Oragenics announced that the stabilized pre-fusion spike protein trimer produced by its Canadian collaborator…
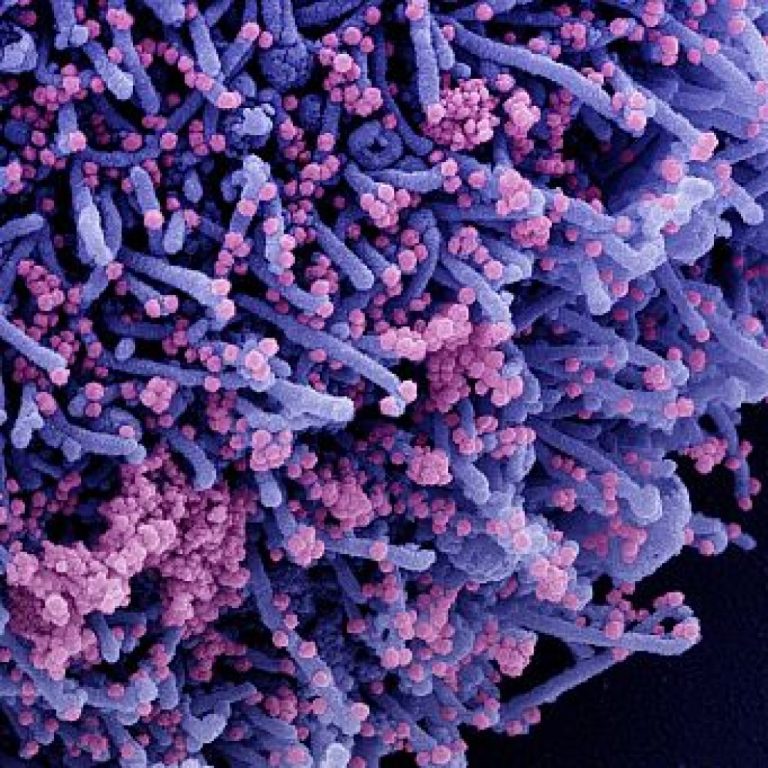
On Aug. 30, 2021, Oragenics announced that the stabilized pre-fusion spike protein trimer produced by its Canadian collaborator…

On Aug. 30, 2021, the Mayo Clinic reported that in an observational study the combination of casirivimab and…

On Aug. 27, 2021, researchers at University of British Columbia’s (UBC) faculty of medicine and BC Cancer Research…

On Aug. 27, 2021, National Aeronautics and Space Administration (NASA) Space station astronauts successfully demonstrated DNA repair in…

On Aug. 26, 2021, Oregon Health & Science University (OHSU) announced a laboratory study found that blood serum…

On Aug. 26, 2021, Pfizer and BioNTech announced the signing of a letter of intent with Eurofarma Laboratorios,…
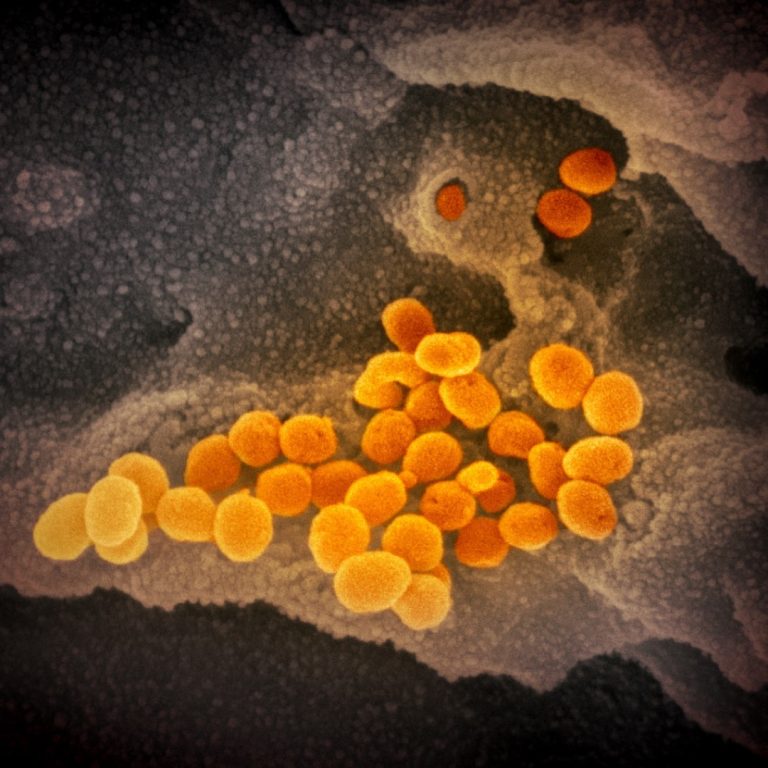
On Aug. 25, 2021, Anixa Biosciences announced that a genomic variant analysis indicateed that its potential compounds may…

On Aug. 25, 2021, Pfizer and BioNTech announced the initiation of a supplemental Biologics License Application (sBLA) to…

On Aug. 23, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Aug. 19, 2021, the University of Oxford reported that obtaining two vaccine doses remained the most effective…

On Aug. 19, 2021, Oregon Health & Science University (OHSU) announced that it was part of a randomized,…

On Aug. 17, 2021, researchers reported the delta variant of the virus that causes COVID-19 was not particularly…

On Aug. 13, 2021, Merck announced that the U.S. Food and Drug Administration (FDA) had approved WELIREG, an…

On Aug. 12, 2021, Innovation Pharma released information regarding the status of its randomized, double-blind, placebo-controlled Phase 2…

On Aug. 11, 2021, the World Health Organization (WHO) announced the next phase in its Solidarity trial that…

On Aug. 3, 2021, CytoDyn announced that Brazil’s regulatory authority ANVISA (Agência Nacional de Vigilância Sanitária) has approved…

On Jul. 29, 2021, Incyte announced the U.S. Food and Drug Administration (FDA) had broadened the Emergency Use…

On Jul. 29, 2021, BOTOX(R) Allergan, an AbbVie company, announced that the U.S. Food and Drug Administration (FDA)…
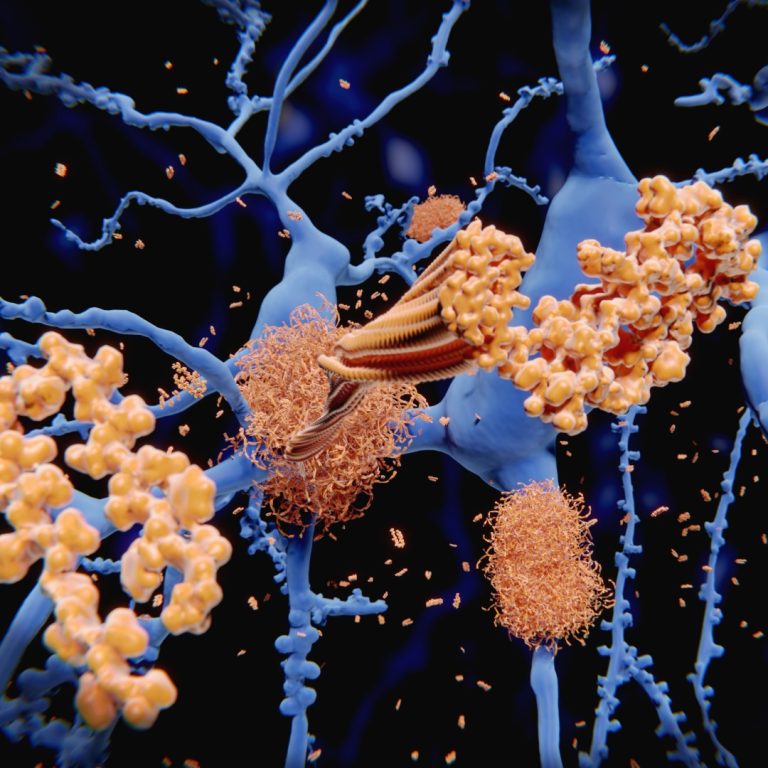
On Jul. 29, 2021, the National Institutes of Health announced it had launched a new online research tool…
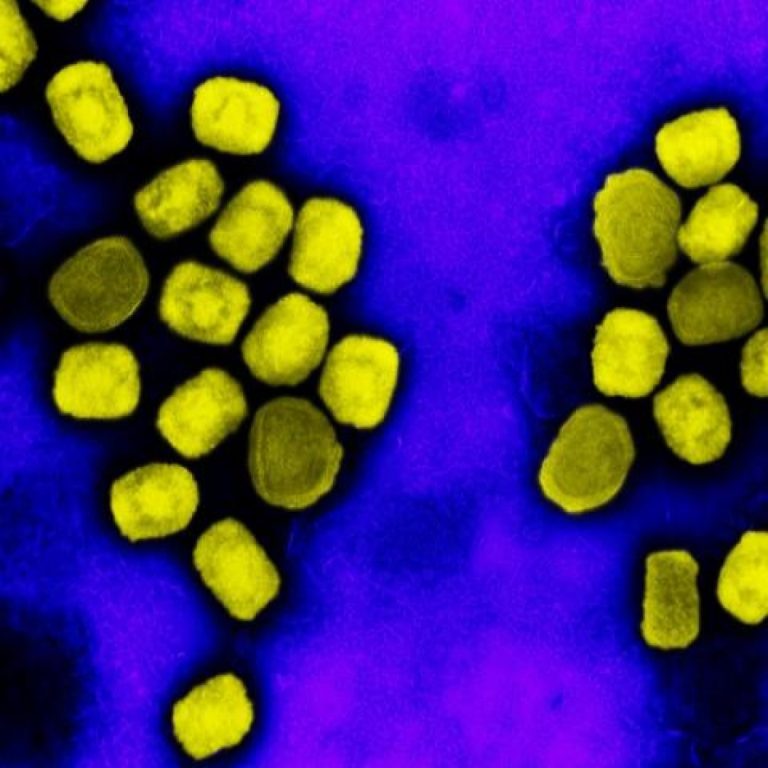
On Jul. 29, 2021, SIGA Technologies announced that it had entered into a collaboration with Oxford University in…

On Jul. 29, 2021, Cocrystal Pharma announced that its SARS-CoV-2 3CL protease lead CDI-45205 and several analogs showed…
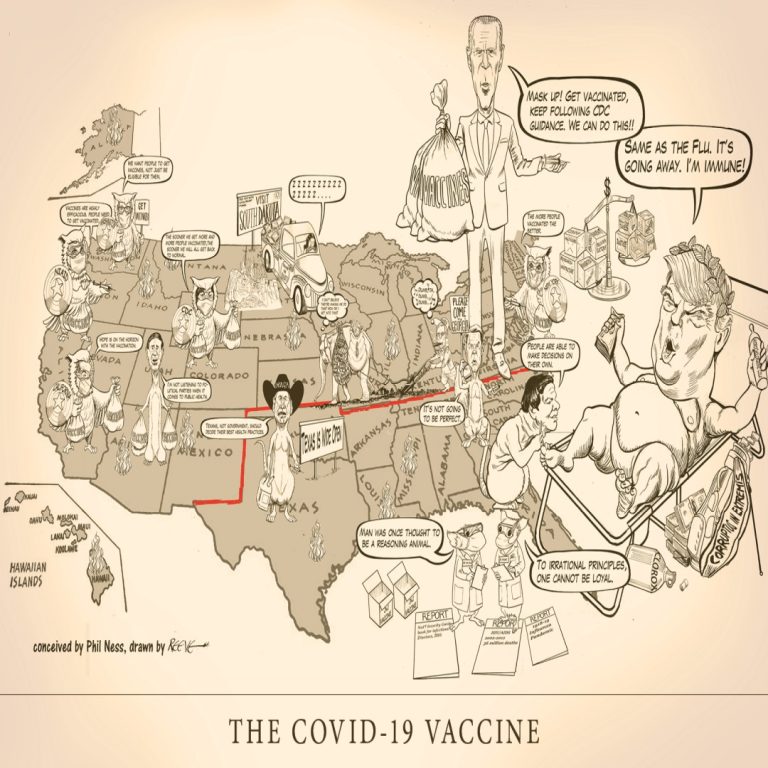
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…
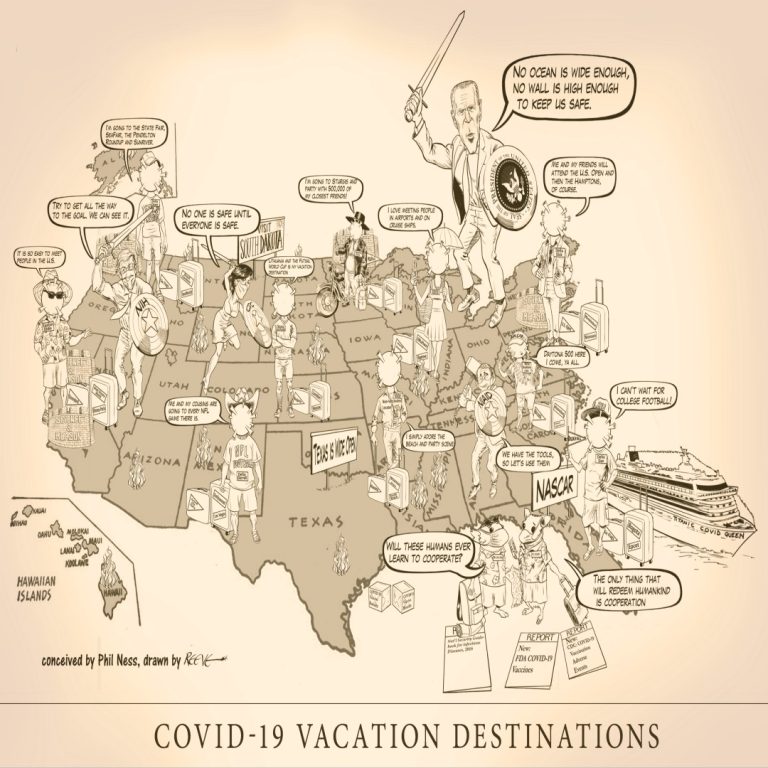
Planning a Summer vacation? Where to go? How about going to one of those large events with tens…
On Jul. 22, 2021, Moderna announced a new supply agreement with Taiwan for 20 million doses of Moderna’s…

On Jul. 16, 2021, Merck announced the U.S. Food and Drug Administration (FDA) had approved VAXNEUVANCE (Pneumococcal 15-valent…
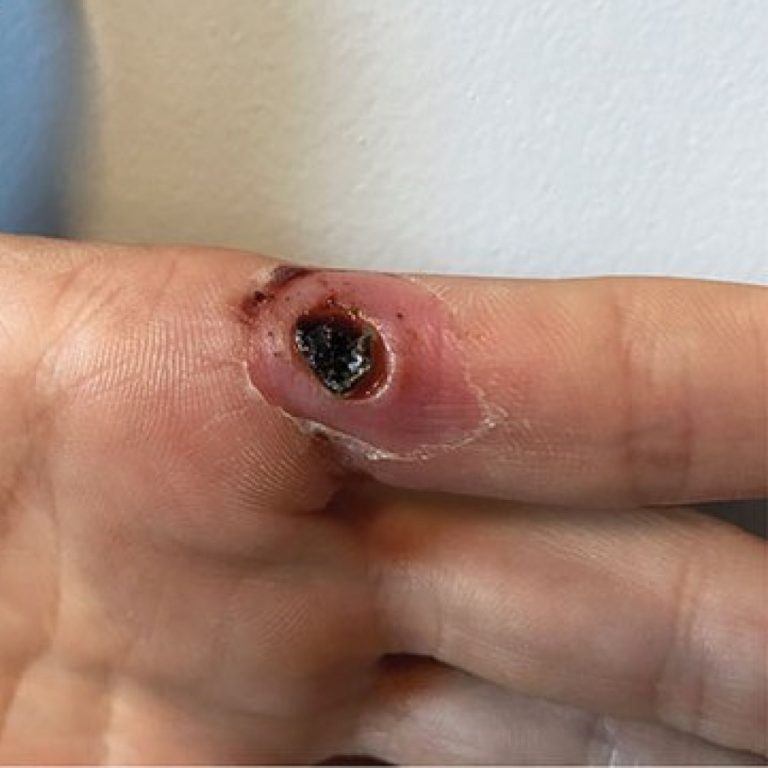
On Jul. 16, 2021, the U.S. Centers for Disease Control and Prevention (CDC) announced and the Texas Department…
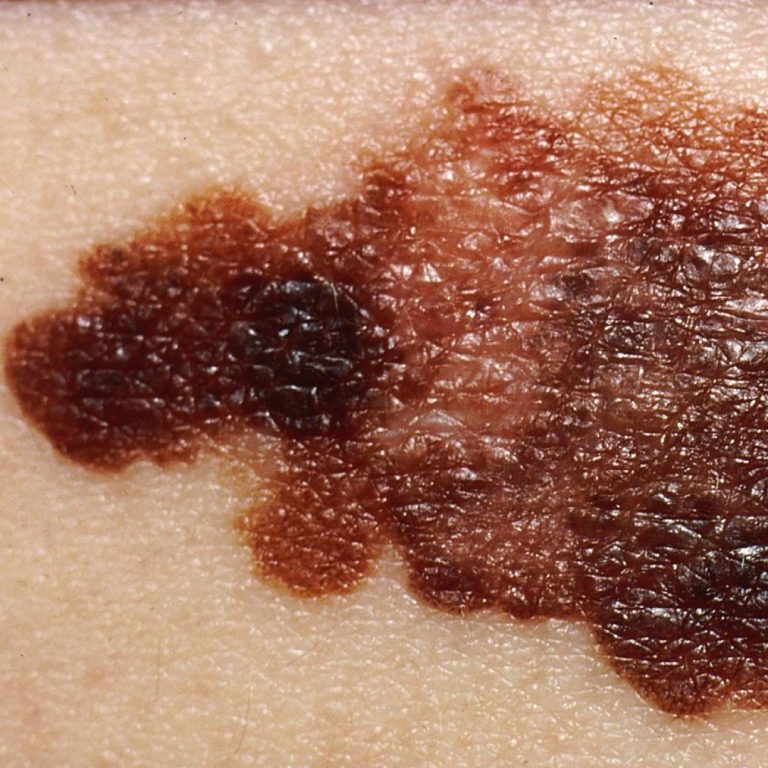
On Jul. 8, 2021, the National Institutes of Health (NIH) reported that the overall cancer death rates continue…

On Jul. 8, 2021, access to the world’s largest browsable resource linking rare protein-coding genetic variants to human…
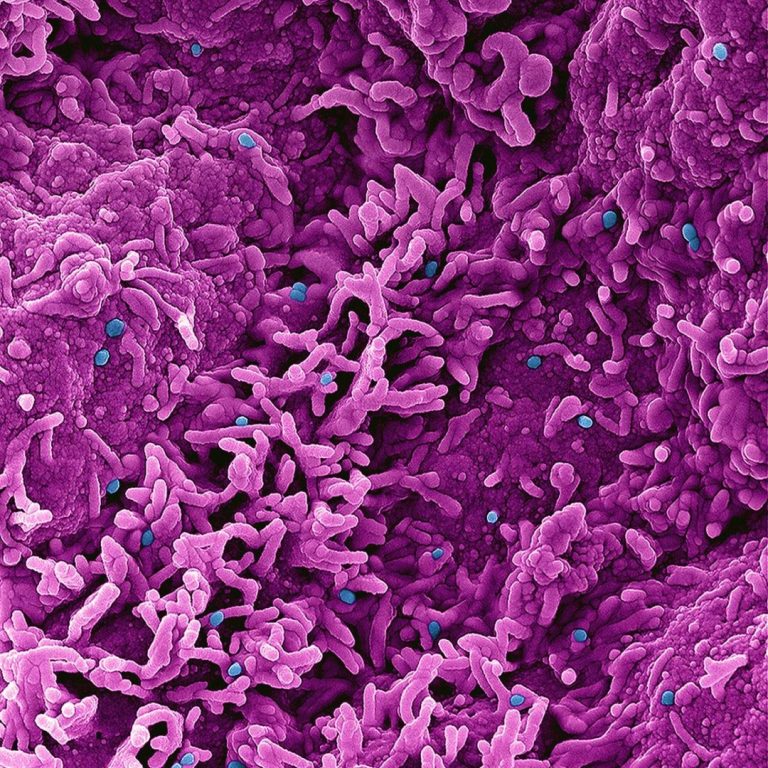
On Jun. 30, 2021, SIGA Technologies announced that it had provided TPOXX (tecovirimat) to Liverpool University Hospitals NHS…
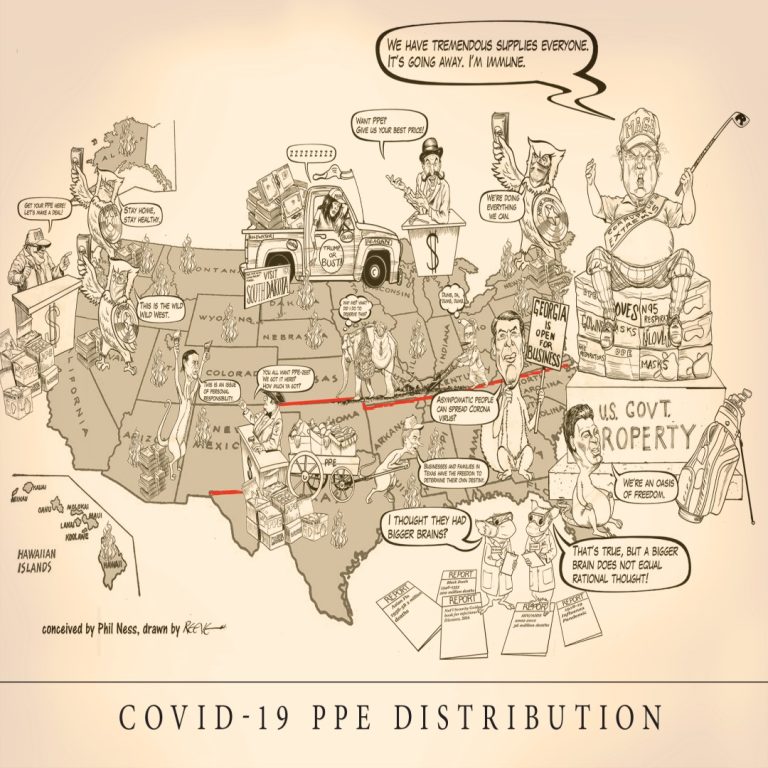
Cast of Characters: Jay Inslee, Governor, Washington | Gavin Newsom, Governor, California | Doug Ducey, Governor, Arizona |…